Benefits of Understanding Feto-Maternal Immune Cross Talk
Speaker: Prof. Dr. Petra Arck
Summery
The body of a pregnant woman undergoes significant changes during pregnancy, both visible and somewhat invisible. The ‘invisible changes’ include that the maternal immune system mounts tolerance to the fetus. This is needed, as the fetus inherits markers from the father that are expressed on the cell surface. These markers, they are called antigens, could provoke a response of the maternal immune systems, similar to the reaction to a mismatching transplant. However, due to the tailored adaptations of the maternal immune system during pregnancy, a rejection of the fetus does not occur. During this adaptation, pregnancy hormones and immune cells are engaged in an intense dialogue. This cross talk between maternal hormones and immune cells ensures that the unborn child fetus can successfully develop until term.
Concomitantly, this maternal adaptation to pregnancy bears significant advantages. It can improve the activity of preexisting maternal autoimmunity. Autoimmunity means that the immune system attacks structures of the own body, for example against brain tissue, as seen in female patients with Multiple sclerosis (MS). In fact, no medication is currently available that has the same strength of ameliorating MS symptoms as pregnancy. This is why pregnancy can be seen as Mother Nature’s top model of understanding autoimmunity. On the other hand, pregnant women are at a significant health disadvantage, as they are more vulnerable to infections, for example with the flu virus. Having the flu during pregnancy can be very dangerous, if not fatal, for the mother and also the unborn child.
Besides these consequences of pregnancy for maternal health, the origin of health or disease in later life of the children can also lie in the period before birth. Challenges during pregnancy, for example maternal stress perception and medication, can cause disadvantages for children’s health later in life. These disadvantages become evident as a weak immunity, which means a poor response to vaccination and a high risk for early life infections. Also, these challenges during pregnancy can cause a greater risk for the children to suffer from chronic immune diseases like allergies and asthma, but also MS or diabetes.
These advantages and disadvantages for mother and child that are linked to pregnancy have been observed in population studies. However, the particular cells and hormones that are modulated during pregnancy and subsequently account for these advantages and disadvantages are still largely unknown. One reason for this limitation is that a number of different medical disciplines are involved to fully understand how this feto-maternal cross talk is operational. In the , we have overcome this limitation by allying clinicians and basic scientists from different medical disciplines, all based at the Medical Faculty of the University of Hamburg and the Heinrich-Pette-Institute, a Leibniz Institute for Experimental Virology.
A total of 21 group leaders and their teams now jointly address two aims. First, we want to identify how the finely tuned balance of immune cells and hormones, activated during the maternal adaptation to pregnancy, can tilt towards advantageous or disadvantageous consequences for maternal health. Second, we seek to understand how the tilt of this balance in response to prenatal stress or medication is disadvantageous for unborn child and leads to an increased risk for immune diseases later in life of these children. To address these aims, we use basic science approaches in research laboratories, combined with the evaluation of immune cells and hormones in the blood of pregnant women or in cord blood taken after birth.
We expect that our findings will lay the foundation for biomarker discovery that may help to induce a ‘pregnancy-like’ immune situation in order to treat patients with MS. Further, we want to reduce the risk for pregnant women and their unborn children to suffer from severe infections. Lastly, we seek to develop guidelines for the identification of unborn or newborn children at risk for immune diseases later in life and development strategies to reduce the risk for these children before or around birth.

- Speaker
Individual Projects of the KFO 296
-
Why does pregnancy improve symptoms of multiple sclerosis?
WHY DOES PREGNANCY IMPROVE SYMPTOMS OF MULTIPLE SCLEROSIS?
Mounting evidence suggests that the required immune regulation during pregnancy is not simply a general ‘immunosuppressive state’, but modulates the way the adaptive immune system, specifically T cells, respond to self and foreign antigens. Intriguing clues to the importance of this immune modulation come from clinical observations in autoimmune diseases including multiple sclerosis (MS). Several studies have shown that inflammatory disease activity and risk for MS relapses decreases throughout pregnancy, but re-bounds after delivery. The reduction of relapse rate in the third trimester is estimated to reach up to 80%, which is a far larger reduction in disease activity than that achieved by currently available MS therapies. Interestingly, in the MS animal model, experimental autoimmune encephalomyelitis (in short EAE), pregnant mice are also protected from disease but show exacerbated neuroinflammation after delivery.
The aim of this project is to understand the mechanisms responsible for pregnancy mediated immune regulation and its benefit on maternal autoimmunity. Based on our preliminary data, we hypothesize that the pathways mediating tolerance against fetal antigens during pregnancy also affect responses against self-antigens, thereby ameliorating autoimmune diseases. Driven by cell-mediated and/or hormonal regulation, this is presumed to result in a shift in the maternal T cell repertoire and temporary induction of antigen-specific immune tolerance.
To test this hypothesis, our translational project is subdivided into three main aims, each of which will be explored in the animal model EAE as well as a clinical prospective cohort of female MS patients over the course of pregnancy and postpartum.
Firstly, we will explore clonal composition of the T cell repertoire as well as characterize in detail self- and foreign-reactive T cell responses during pregnancy using a combination of next generation sequencing of the T cell repertoire, and functional probing of antigen-specific T cell responses. In parallel and with similar methods, we will assess the mouse T cell repertoire in pregnant, post-pregnant and virgin mice with EAE and healthy controls.
Secondly, we want to identify relevant major T cell subpopulations mediating pregnancy-associated advantages for maternal health. Based on the results of our first objective, we will study the most strongly regulated T cell subsets by performing in depth flow cytometric phenotyping. Again, this will be done similarly in human and mouse samples. Moreover the mouse model will allow us to examine the antigen specificity and the functionality of these T cell subsets in more detail.
Thirdly, we want to investigate the interaction of pregnancy-related hormones with self-reactive and foreign-reactive T cell responses. To assess the effect of pregnancy-related hormones on differentially regulated T cell subsets throughout pregnancy in both the mouse model and human samples, single cell analysis will be used to quantify the gene expression of T cell differentiation, T cell activation and steroid signaling. Finally, cell-specific steroid receptor knock-out mice will be used to compare frequency, phenotype and function of T cell subpopulations in pregnant and non-pregnant EAE animals.
This translational project will complement the projects "WHY DO WOMEN DEVELOP SEVERE INFLUENZA ILLNESS DURING PREGNANCY?" and "INFLUENCE OF PREGNANCY-RELATED HORMONES ON IMMUNITY" to provide a comprehensive picture of the “costs and benefits” of feto-maternal immune cross talk and maternal health. In addition, we will strongly depend on the Service project for obtaining the samples from healthy pregnant women matched to our MS cohort for age of the mother, parity, and sex of the child.
/bilder/personen/friese_manuel_202209080001_1_kontaktbild.jpg) Prof. Dr. med.Manuel A. Friese
Prof. Dr. med.Manuel A. Friese- Director of the institute
- Center for Molecular Neurobiology Hamburg (ZMNH)
- Institute of Neuroimmunology and Multiple Sclerosis
- Medical Specialist in Neurology
Contact
Location
Falkenried 94, 20251 Hamburg , Room number 2_63 Prof. Dr.Stefan Gold
Prof. Dr.Stefan Gold- Project leader
Contact
PhoneTelefaxLocation
Falkenried 94, 20251 Hamburg , 2nd Floor, Room number 63 Nina Heckmann
Nina Heckmann- Project member
Location
Falkenried 94, 20251 Hamburg , 2nd Floor Dr. rer. Biol. Hum.“Caren Ramien
Dr. rer. Biol. Hum.“Caren Ramien- Research consultant
Location
Main Building O10 , 2nd Floor, Room number 02.2.66.1 -
Why do women develop severe influenza illness during pregnancy?
WHY DO WOMEN DEVELOP SEVERE INFLUENZA DURING PREGNANCY?
Pregnant women are the highest risk group to develop severe influenza illness and even can die from it. This disadvantage for maternal health has been observed during so-called pandemics, the last one known as Mexican or Swine flu in 2009. Here, women had a 5 times higher risk to be admitted to hospital, compared to non-pregnant women. Therefore, vaccine recommendations have been revised worldwide and the World Health Organization now recommends vaccination of pregnant women as a first priority.
The mechanisms underlying this increased influenza risk for pregnant women are still largely unknown. Therefore, we aim to understand whether the influenza virus may be altered when a pregnant individual is infected. We also want to understand how the maternal immune system during pregnancy responds to the influenza virus and compare this to the immune response of non-pregnant individuals.
The risk to suffer from influenza during pregnancy can be reduced, if not prevented, by a vaccination against influenza. Surprisingly, only a small percentage of women during their reproductive years take advantage of this prevention method. Therefore, we anticipate that a better understanding of how pregnant individuals are at higher risk to suffer from severe influenza will increase the threat awareness of women during their reproductive years and therefore will contribute to a higher vaccination rate. Moreover, we expect that our identification of why the immune response to eliminate the influenza virus during pregnancy is weak will also be of relevance for other groups of people with a high influenza risk, such as the elderly, children, or patients taking immunosuppressive drugs.
 Prof. Dr.Gülsah Gabriel
Prof. Dr.Gülsah Gabriel- Project leader
Contact
PhoneTelefaxLocation
HPI, 1st Floor, Room number 111 Prof. Dr. med.Petra ArckMD
Prof. Dr. med.Petra ArckMD- Project leader
Location
Campus Forschung N27 , 4th Floor, Room number 04.039 Location
Location
Campus Forschung N27 , 2nd Floor, Room number 02.057 Alexandra Hierweger
Alexandra Hierweger- Project member
Henning JacobsenM. Sc.Location
HPI, 1st Floor -
Stress during pregnancy – consequences for the child later in life?
STRESS DURING PREGNANCY – CONSEQUENCES FOR CHILDREN IN LIFE
Prenatal stress challenge has been linked to disadvantages for children's health later in life, such as an increased risk for chronic immune diseases. The immune system largely develops during fetal life and hence, fetal immune organs may be a vulnerable for prenatal stress challenges. In turn, the impaired fetal immune development may become clinically evident during childhood by the onset of a chronic immune disease. Despite this growing recognition of an increased risk for chronic immune diseases in children in response to prenatal stress, the identification of pathways causally involved in this association is largely missing. This was the motivation for us to develop the present project. We here focus on the role of key stress hormones and glucocorticoids on fetal immune and organ development.
The fetus requires glucocorticoids in order to ensure structural and functional development of organs, such as the lung or immune organs. Surprisingly, until late in gestation, the fetus itself is not capable of producing glucocorticoids on its own. Therefore, maternal glucocorticoids are transported via the placenta to allow fetal development even in the absence of fetal glucocorticoid production.
However, the fetus must also be protected from overshooting maternal glucocorticoids, which are secreted in response to stress challenge. A placental enzyme called 11β-hydroxysteroid dehydrogenase type 2 (11βHSD-2) acts as a ‘gatekeeper’ in order to protect the fetus.
Given the importance of this ‘gatekeeper’, what happens if 11βHSD-2 function is failing? This is what we aim to address in the present project. We seek to analyze mice that are genetically engineered to lack 11βHSD-2 exclusively in the placenta. This means that the fetus is unprotected from maternal glucocorticoids. This approach allows us to test the impact of overshooting glucocorticoids on fetal development compared to control mice. Moreover, we seek to understand the role of pregnancy hormones, for example progesterone, on strengthening the gatekeeper function of 11βHSD-2. We will compare our findings of glucocorticoids produced by the mother, the so-called endogenous glucocorticoids, on fetal development to the impact of exogenously given glucocorticoids and other medications taken during pregnancy, which are studied in projects titled “pain relievers during pregnancy” and “prenatal steroids”.
Our basic science approach involving mice allows us to dissect the importance of normal and extremely high levels of glucocorticoids on fetal development. In close collaboration with the Prenatal Determinants of Children’s Health study, in short PRINCE study ( www.uke.de/prince ), we will then be able to understand the relevance of our insights from mouse models for humans. We will test how mothers with high levels of stress perception and associated high levels of glucocorticoids can be identified. Once this is possible, we seek to develop strategies to prevent human fetuses from high glucocorticoid expose and risk for immune diseases later in life.
 Prof. Dr. med.Petra ArckMD
Prof. Dr. med.Petra ArckMD- Project leader
Location
Campus Forschung N27 , 4th Floor, Room number 04.039 Dimitra ZazaraMD
Dimitra ZazaraMD- Junior research group leader
- Assistenzärztin in Weiterbildung
- Assistant Physician
Location
Campus Forschung N27 , 3rd Floor, Room number 03.034 -
Pain relievers during pregnancy: A double edged sword
PAIN RELIEVERS DURING PREGNANCY: A DOUBLE EDGED SWORD
While fever and general pain are debilitating especially during pregnancy, medication might come along with long-term side effects for the child. Although a multitude of pain relievers are available, only few are considered to be safe during pregnancy. The most recommended agent is N-acetyl-p-aminophenol, acetaminophen, or APAP in short. It has been firstly used in 1893 and subsequently became a first-line medication for treatment of pain and fever without any obvious complications.
APAP is an OTC drug, meaning that it is available without prescription by a physician. Presently it is sold under different brand names, pure or in combination with other active compounds like vitamin C or caffeine. Since the 1990ies, APAP fell into disrepute because product label confusion resulted in unintentional overdosing, leading to cases of severe acute liver failure, especially in the US.
However, being apparently safe at therapeutic doses and being neither an opioid nor an inhibitor of coagulation, APAP remains the first choice to manage fever and pain during pregnancy.So, why do we care about this obviously helpful drug?
Recent scientific studies showed, that APAP does more than relieving pain and reducing fever. The compound is able to alter our immune response. Being pregnant and taking this drug bears the risk of causing alterations in the immune system of the unborn child, too. This may lead to long term consequences like increased risk for asthma and probably other auto-immune diseases in the child. Some of the symptoms might show up not before their 7th birthday.
With this knowledge we want to learn more about the mechanisms APAP works and especially how exactly our immune system is affected. In recent studies we showed that hormone levels were altered and that stem cell numbers in the developing child were reduced.
It is yet unknown how exactly APAP interacts with the developing fetal organs and why those long-term consequences might develop. Our project with focus on pain relievers during pregnancy therefore aims at developing a better understanding of how cells in pregnant women and their children react when they are treated with APAP.
We are following a modern bench-to-bedside strategy, designing models and experiments to mimic the complicated processes during pregnancy as close as possible. We are carefully analyzing the impact of APAP and its well-known toxic metabolite N-acetyl-p-benzoquinone imine (NAPQI) specifically looking at changes occurring in blood cells and in tissue. Using high tech multicolor flow cytometry we aim to identify different stem cells and immune cells, which are likely to be affected by the drug.
Our approach is embedded in harnessing the scientific ability of a consortium of partners within and outside the . Discussing ideas, conducting experiments and exchanging insights, in particular with the other projects dealing with prenatal challenges like endogenous and exogenous glucocorticoids, as well as the enables us to shed light on stem cell biology and immune during pregnancy. This will help us to understand how APAP affects the pregnant mother, the developing baby, and in the children later in life.
Combining our data we want to derive a recommendation for APAP medication for pregnant women and their physicians.If you have any questions regarding this project feel free to contact us.
 Laura Katharina Berkhout
Laura Katharina Berkhout- Doctoral student
PhoneE-mail -
Prenatal steroids
PRENATAL STEROIDS
Over the past 30 years, prenatal corticosteroid treatment, called betamethasone has been administered to thousands of women at risk of premature birth to accelerate fetal lung maturation in the preterm neonates. A remarkably higher survival-rate of premature and infants with very low birth-weight, highlights the efficiency of this treatment. However, the long term effects have not been analyzed in detail, especially the possible effects on the immune system. This is very important to know considering that other steroids are known to induce cell death of developing T cells.
The development of the immune system of mammals begins before birth. The thymus is an essential organ for T cell development and establishment of tolerance. At birth, thymic function is at its peak and therefore events affecting the thymus may have consequences for the child’s immunity as immature thymocytes are extremely sensitive to steroids. Injection of physiological doses of betamethasone has been shown to result in a dramatic reduction of the thymus volume and death of T cell precursors in our model . The lost niche is quickly replenished, and this accelerated maturation may result in errors during selection events. Of notable significance, in vitro treatment of human thymocytes with low betamethasone doses has also been shown to induce cell death of the developing cells.
Western Societies have been faced with an unparalleled increase in the incidence of autoimmune diseases and asthma over the past decades. In this context it is particularly noteworthy that the age at which diseases like type I diabetes occur is decreasing, and in many cases occur with more severe course, when compared to a few decades ago. Recent evidence indicates that the exposure to prenatal steroids is a risk factor for neonatal sepsis and for developing asthma and diabetes later in life. Given the importance of T cells in adaptive immune responses, we hypothesise that prenatal steroid treatment impairs the normal development of the offspring’s immune system and therefore increases the risk for autoreactivity/allergy later in life.
The main focus of our project is to study T cell development and long-term effects on the immune system in offspring whose mothers were treated with prenatal corticosteroids. To test our hypothesis we plan to apply our model of autoimmunity and allergy, and to analyse in detail the immune system at birth and later in life of children whose mothers received steroids.
In consideration of that half of the women receiving prenatal steroids have not delivered by the time that the beneficial effect of steroids wears out, awareness of harmful long term effects could lead to a re-evaluation of treatment protocols.
 Prof. Dr.Eva Tolosa
Prof. Dr.Eva Tolosa- Head of research group
- Project leader
Location
Campus Forschung N27 , 2nd Floor, Room number 02.055 -
Diet during pregnacy and maternal obesity - Effects on subsequent offspring health
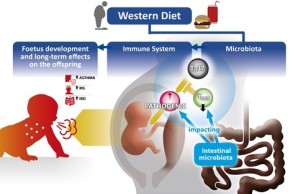
Unprecedented consumption of high sugar, fat and salt dietary components, commonly referred to as „Western“ diet, is currently observed not only in developed countries, but also in developing countries and is considered one of the leading causes of obesity. It is apparent that a growing number of individuals are “forced” to consume this diet due to socioeconomic circumstances. In the year 2014, according to the World health organization, 600 million adults suffered from obesity. Many of them were also diagnosed with related metabolic or cardiovascular diseases. Interestingly, obesity is often associated with chronic and pathological activity of the immune system. Furthermore, epidemiological data indicate that consumption of Western diet during pregnancy and maternal obesity is often associated with chronic and pathological activity of the immune development and account for poor children’s health later in life.
This project aims to elucidate the cellular and molecular mechanisms underlying these effects. The main hypothesis is that Western diet and maternal obesity skew the maternal immune adaption to pregnancy, especially the CD4 T cell response required to mount tolerance towards the semi-allogeneic foetus towards inflammation. In turn, this inflammation leads to impaired foetal development and has a pivotal impact on the compositon of the intestinal mircrobiota, which in turn is in continuous cross tall with the immune system, we will test if diet-mediated inflammation is mediated by the Western diet mediated imbalance of the maternal microbiota.
Novel multi-cytokine fate reporter and conditional knock out mouse models will be used to test if maternal Western diet affects foetal development and offspring’s immunity via an altered function of distinct CD4 T cell subsets. To understand the molecular details of diet-induced changes in these mice, we will employ single cell trancriptomics, epigenetics, and state-of-the-art computational approaches. To translate the mechanistic insights from the mouse models into similarities and differences in humans, we will use data and biological samples from the prospetive pregnancy cohort PRINCE. Our project will help to identify new therapeutic targets such as pathogenic CD4 T cells, to develop new therapies, which aim to prevent the offspring having to suffer life-long health problems due to the social circumstances in which they are born.
Prof. Dr.Nicola Gagliani- Head of research group
Location
Campus Forschung N27 , 3rd FloorProf. Dr.Stefan Bonn- Director of the institute
Location
Falkenried 94, 20251 Hamburg , Room number 1.58 -
Congenital Cytomegalovirus infection
Congenital Cytomegalovirus infection
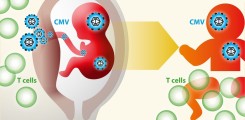
Cytomegalovirus (CMV) is a large double-stranded DNA virus and member of the Herpesvirus family. It is highly prevalent worldwide, with seroprevalence rates ranging from 40 to 100%. Primary infection is usually subclinical in healthy adults due to the control by the immune system. T lymphocytes are known to play a critical role in the control of CMV infection. However, the antiviral immune response cannot eliminate the virus, nor can it reliably prevent superinfection with additional CMV strains or reactivation of the latent infection.
Importantly, congenital infection with CMV in newborns is the most frequent cause of permanent disabilities including neurological damage, such as sensorineural hearing loss and mental retardation. Approximately 1% of children worldwide are born with congenital CMV infection, and ~10% of them are clinically symptomatic at birth and likely to suffer from long-term sequelae. Another 5-10% develop late-onset disabilities.
The factors determining vertical transmission from mother to foetus and the symptomatic versus asymptomatic outcome of congenital or neonatal CMV infection remain unknown. Additionally, there is no vaccine available and the only accepted treatment with Valganciclovir carries the risk of serious adverse effects.
In this project we aim to investigate factors that could increase the risk of prenatal CMV infection and severity of neonatal disease. We will determine the role of prenatal challenges, the anti-CMV T cell response of mother and child, and viral pathogenicity factors. We will investigate if maternal stress and medication intake during pregnancy – challenges which are potentially avoidable – render the offspring more prone to develop a severe course of early life CMV infection. In depth characterisation of maternal and neonatal antigen-specific T cells will define their role in control of CMV infection during the early life period. Finally, we will investigate the pathogen itself to identify viral genetic factors that determine its pathogenicity and likelihood of vertical transmission. In summary, we intend to answer pending questions of CMV infection biology that could lay the basis for new diagnostic or therapeutic approaches.
Prof. Dr. Wolfram Brune
Head of Research Department Virus-Host Interaction
Heinrich Pette Institute
Leibniz Institute for Experimental Virology
Martinistr. 52
20251 Hamburg
Germany Prof. Dr. med.Felix StahlPhD
Prof. Dr. med.Felix StahlPhD- Medical specialist
- Principal investigator
- Medical Specialist in Laboratory Medicine
Contact
PhoneTelefaxCellphoneE-mailLocation
Campus Forschung N27 , 2nd Floor, Room number 02.054 -
PRINCE Study - Prospective birth cohort "Prenatal determinants of children`s health"
Current medical practice falls short to prenatally identify children at risk for chronic immune diseases later although a growing body of evidence provides a link between prenatal challenges and occurrence of chronic immune disease in postnatal life. Our research focuses primarily on the interactions between environmental factors to which a woman is exposed to during pregnancy and the risk for the child to develop chronic immune diseases such as allergies and asthma later in life.
Our aim is to identify markers that allow to monitor an undisturbed adaptation of the mother to pregnancy, along with a normal development of the fetal organs, including key organs of the immune system, that could help to identify fetuses at risk.
To achieve this aim, we recruit of pregnant women into the (Prenatal Determinants of Children’s Health). The PRINCE study is a prospective, longitudinal population based cohort study of pregnant women and their children. It serves as a central pillar to the clinical research unit 296. We document environmental cues (nutrition, stress perception, medication, vaccination an others), as these factors may interfere with maternal adaptation to pregnancy and fetal development. We also collect biological samples to identify markers for an appropriate adaptation to pregnancy. These samples are kept following standardized tissue banking procedures and transparently distributed within the network’s research projects. We also ensure that highest technical standards for tissue and clinical analyses are applied. This will allow us to gain an understanding of functional pathways through which environmental cues affect fetal development. We envision that the insights arising from this translational research will enable us to develop clinically relevant "evidence-based-guidelines" for health related behavior that can be applied routinely in clinical maternity care. Identifying the fetus at risk early during pregnancy holds the potential for improvement or prevention of decease.
The PRINCE pregnancy cohort provides the healthy human reference population needed for projects of challenged human and mice pregnancies.
Because PRINCE is harmonized with already existing birth cohorts (Life Child Birth Cohort, the Raine Cohort, the MAS study and others) its results contribute to a much larger international data pool. Data can thus be shared in future collaborations.
Clinical Coordinator of the PRINCE Study
 Prof. Dr. med.Anke Diemert
Prof. Dr. med.Anke Diemert- Senior physician
- Medical Specialist in Obstetrics and Gynecology
- Special Midwifery and Perinatal Medicine
- DEGUM II
Contact
PhoneTelefaxCellphoneE-mailLocation
Main Building O10 , 2nd FloorContact
 Prof. Dr. med.Petra ArckMD
Prof. Dr. med.Petra ArckMD- Project leader
Location
Campus Forschung N27 , 4th Floor, Room number 04.039 Prof. Dr. med.Kurt Hecher
Prof. Dr. med.Kurt Hecher- Medical Specialist in Obstetrics and Gynecology
- Special Midwifery and Perinatal Medicine
- DEGUM III
Contact
PhoneTelefaxE-mailLocation
Main Building O10 , 4th Floor Univ.-Prof. Dr. med.Ania C. Muntau
Univ.-Prof. Dr. med.Ania C. Muntau- Director of the department
- Medical Specialist in Pediatrics and Youth Medicine
Location
O45 , 1st Floor, Room number 01.5.014.1 Prof. Dr. med.Dominique Singer
Prof. Dr. med.Dominique Singer- Head of medical management
- Medical Specialist in Physiology
- Medical Specialist in Pediatrics and Youth Medicine
- Special Pediatric Intensive Care
- Neonatology
Location
O45 , 1st Floor Location
Location
Campus Forschung N27 , 2nd Floor, Room number 02.055 Dr. med.Corinna Kramer
Dr. med.Corinna Kramer- Physician
Location
O45 , Ground Floor, Room number 00.5.012.1 Dr. med.Mirja Pagenkemper
Dr. med.Mirja Pagenkemper- Project member
- Medical Specialist in Obstetrics and Gynecology
Location
Main Building O10 , 5th Floor Gudula HansenM. Sc. Health Services Management
Gudula HansenM. Sc. Health Services Management- Nutritionist
Contact
PhoneTelefaxCellphoneE-mailLocation
Main Building O10 , 2nd Floor, Room number 02.8.076.1