LARGE PUBLIC-PRIVATE CONSORTIUM
The ELBS is a dynamic network consisting of partners from academia and industry with the common goal of bringing LB tests into clinical routine. The network covers all relevant areas to reach this goal, ranging from harmonization of technologies, to the design of suitable clinical studies, and the interaction with regulatory bodies. The combination of the collective expertize and knowledge of the ELBS members with the strategic implementation of joint projects makes the ELBS a uniquely suited driver and valuable partner in the area of LB.
CONSORTIUM SIZE AND DIVERSITY
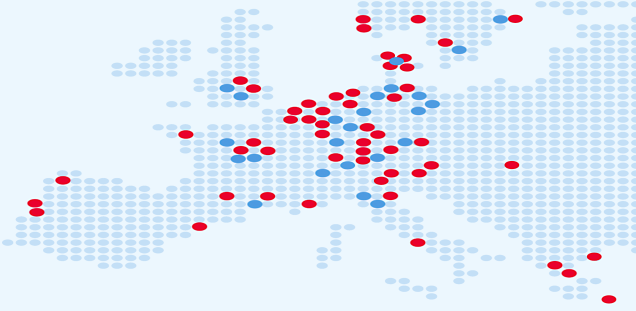
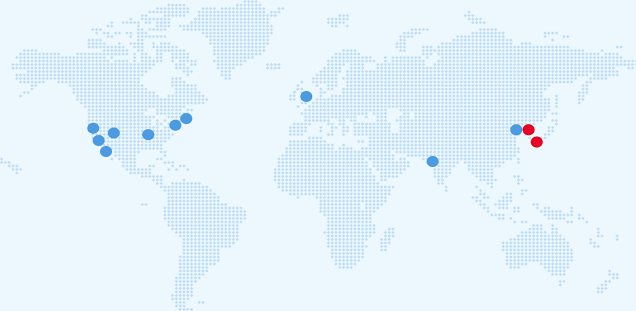
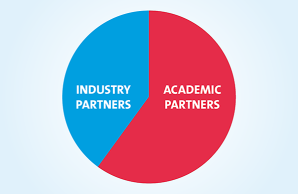
The ELBS network currently consists of more than 87 member institutions with more joining each year. About 60 percent of our members are academic institutions or university hospitals; About 40 percent represent industry partners spread across biotech, device manufacturers, diagnostic companies, standard providers and pharmaceutical companies. The ELBS member institutions also span three continents (Asia, Europe, USA), the majority of members being located in Europe.
HARMONIZATION AND STANDARDIZATION
In order to work towards robust LB tests that are applicable in a clinical setting, standardization and harmonization of preanalytical as well as analytical parameters are essential. One valuable approach to achieve such harmonization is the formulation of specific guidelines. Another is performing actual wet lab work including ring experiments and technology benchmarking. The ELBS includes both complementary approaches in its activity profile.
COMPLEMENTARY FOCUS AREAS BY DIFFERENT WORKING GROUPS
In order to move the field of LB forward and in line with our holistic approach, the ELBS consists of distinct working groups that combine specific areas of expertize. The working groups (WGs) are Dissemination & Education, Clinical, Technology, and Regulatory Issues. Each WG is chaired by a steering committee of 2 – 4 members that drive the activity of the network in their specific area of expertize. More information on the WGs can be found under the respective links below.
ELBS WORKING GROUPS
-
WORK GROUP DISSEMINATION & EDUCATION
The goal of the Education & Dissemination WG is to build a strong and interconnected network with all essential players on board.
This is achieved by:
- Establishing connections to other scientific societies and initiatives in the liquid biopsy space
- Generating awareness of liquid biopsy applications for researchers, clinicians and others
- Increasing knowledge in relevant areas of the LB field (through workshops, white papers, etc.)
- Online representation
LEADS - Establishing connections to other scientific societies and initiatives in the liquid biopsy space
-
WORK GROUP CLINICAL
The goal of the Clinical WG is to translate clinical insights on liquid biopsy applications into clinical utility.
This is achieved by:
- Identification of urgent clinical needs (markers and assays)
- Identification of relevant projects from a clinical perspective
- Providing guidance and a clinical perspective for the other WGs
- Defining good practice criteria for inclusion of LB in clinical trials
- Revising and designing “ELBS approved” clinical trials in the realm of LB
LEADS - Identification of urgent clinical needs (markers and assays)
-
WORK GROUP TECHNOLOGY
The technology working groups are subdivided according to their focus. Currently 3 WGs exist spanning the CTC, ctDNA, and EV fields. The overarching goal of these groups is to identify optimally suited assays for specific scientific and clinical questions and to work towards optimization, harmonization and standardization of these methods.
Activities include, but are not limited to:
- Benchmarking novel technologies
- Establishing and improving SOPs
- Organizing ring trials to assess and compare technology proficiency
- Educating on limitations, challenges and strengths of existing analytical approaches
- Supporting interested labs in standardization and certification processes (e.g. ISO 15189)
LEADS
CTC WORK GROUP:
Evi Lianidou, Nikolas Stöcklein and Yong-Jie Lu
ctDNA WORK GROUP:
Ellen Heitzer, Ed Schuuring and Patrizio Giacomini
EV WORK GROUP:
An Hendrix, Franz Ricklefs and Basant Kumar Thakur - Benchmarking novel technologies
-
WORK GROUP REGULATORY
The regulatory WG pursues the overarching goal of driving implementation and the introduction of liquid biopsy assays into routine clinical practice.
This goal is pursued through:
- Acquiring knowledge on regulatory terms and conditions (mainly) in EU countries
- Collecting input and expertize from all stakeholders in health care
- Exchange with regulatory agencies (e.g. EMA, FDA) and health care providers
- Initiation of collective pan-European initiatives to work jointly towards approval and reimbursement of liquid biopsy approaches
LEADS - Acquiring knowledge on regulatory terms and conditions (mainly) in EU countries
INTERSOCIETY WORKING GROUP
-
WORK GROUP ISEV-ELBS INTERSOCIETY
We are pleased to announce that we have founded an Intersociety Working Group together with ISEV.
This working group aims to support the clinical translation of EV-related biomarker research.
HISTORY
The ISEV-ELBS Intersociety Working Group was founded in 2023. ELBS is the European Liquid Biopsy Society, and this working group aims to improve the quality of biobanks used for clinical biomarker exploration, which includes EV-related biomarker research. In the last 20 years, there has been a revolution in early diagnosis, prognosis and therapeutic efficacy of diseases based on information collected from non-invasive liquid biopsies.VISION AND AIMS
Together the ISEV-ELBS Intersociety Working Group aims to foster collaboration between the two societies with the goal of promoting reproducibility of liquid biopsy-EV preparation and analysis and encouraging discussion on EV research and its applications in liquid biopsy approaches. Together the two societies will co-host meetings, develop collaborative initiatives and report developments in the EV-liquid biopsy field. This will foster the development of novel EV biomarkers and their translation to the clinic.
MEET THE CHAIR
Caterina Nardella Senior Scientist and Programme Manager, Laboratory of Computational and Functional Oncology
Department of Cellular, Computational and Integrative Biology (CIBIO)
University of Trento, Italy
caterina.nardella@unitn.it
An Hendrix Full professor, Laboratory of Experimental Cancer Research, Department of Human Structure and Repair, Ghent University, Ghent, Belgium
Principal investigator, Cancer Research Institute Ghent, Ghent, Belgium Founder and President of the Belgian Society for Extracellular Vesicles
an.hendrix@ugent.be
Carlos Salomon Gallo
Professor
Director, UQ Centre for Extracellular Vesicle Nanomedicine
UQ Centre for Clinical Research
Faculty of Medicine
The University of Queensland, Australia
c.salomongallo@uq.edu.au
Julia V. Burnier Director, Liquid Biopsy Unit
Research Institute of the McGill University Health Centre,Montréal, Canada
julia.burnier@mcgill.ca
NEWS: work in progressOUTPUTS: work in progress
EVENTS: work in progress