ACADEMIC PARTNERS
- Aarhus University
- ELBS Team
- Profile Liquid Biopsy Research
-
Aarhus University


Aarhus University was founded in 1928 and is Denmark’s second-largest university, with 38.000 students, 8.400 employees, five faculties, and research activities all over the country. Research and education of the highest international quality are at the core of our mission, and strong partnerships with our society are at the heart of our activities. Curiosity is our driving force right from the students in the library to the researchers at their desks, laboratories, and clinics: We are all driven by the urge to delve into the deep, create in the present, and contribute to the society.
The Department of Clinical Medicine is the largest department at the Faculty of Health and, due to its size, has a very significant position at the faculty within the research-based educational efforts. The department was officially established in 1972, but the experimental clinical research has taken place since 1935, when the first laboratories directly related to the clinical departments were established. We collaborate closely with the regional hospitals - in particular Aarhus University Hospital - and the department’s research facilities are integrated throughout the hospitals where research and clinical education go hand in hand.
-
-
ELBS Team

Claus Lindbjerg Andersen
Professor, Group Leader
Department of Clinical Medicine
Department of Molecular Medicine
Aarhus University and Aarhus University HospitalBrendstrupgaardsvej 21A
8200 Aarhus N
Denmark
Lars Dyrskjøt
Professor, Group Leader
Department of Clinical Medicine
Department of Molecular Medicine
Aarhus University and Aarhus University HospitalBrendstrupgaardsvej 21A
8200 Aarhus N
Denmark
Nicolai Juul Birkbak
Associate Professor, Group Leader
Department of Clinical Medicine
Department of Molecular Medicine
Aarhus University and Aarhus University HospitalBrendstrupgaardsvej 21A
8200 Aarhus N
Denmark
Jakob Skou Pedersen
Professor, Group Leader
Department of Clinical Medicine
Department of Molecular Medicine
Bioinformatics Research Center
Aarhus University and Aarhus University HospitalBrendstrupgaardsvej 21A
8200 Aarhus N
Denmark
Karina Dalsgaard Sørensen
Professor, Group Leader
Department of Clinical Medicine
Department of Molecular Medicine
Aarhus University and Aarhus University HospitalBrendstrupgaardsvej 21A
8200 Aarhus N
Denmark
Iben Lyskjær Heimann
Associate Professor, Group Leader
Department of Clinical Medicine
Department of Molecular Medicine
Aarhus University and Aarhus University HospitalBrendstrupgaardsvej 21A
8200 Aarhus N
Denmark
Karen-Lise Garm Spindler
Clinical Professor
Department of Clinical Medicine
The department of Oncology
Aarhus University and Aarhus University HospitalPalle Juul-Jensens Boulevard 35
8200 Aarhus N
Denmark -
-
Profile Liquid Biopsy Research
Cancer types Colorectal cancer, bladder cancer, anal cancer, prostate cancer, renal cancer among others. Clinical application Early cancer detection, Detection of minimal residual disease, Recurrence surveillance, Treatment response prediction, Treatment response monitoring, Diagnostic pathway improvements. Liquid Biopsy Source Blood, plasma, serum, PBMC, and urine. Technologies available for liquid biopsy Droplet Digital PCR (ddPCR), Targeted Duplex sequencing, Whole genome sequencing and In-house developed pipelines for ctDNA detection based on mutational profiles, fragmentation, epigenetic signatures, and somatic copy number alterations. Biobank Solid tumors – tissue, blood, plasma, serum, PBMC and urine. Key publications Unraveling the potential clinical utility of circulating tumor DNA detection in colorectal cancer-evaluation in a nationwide Danish cohort.
Henriksen TV, Demuth C, Frydendahl A, Nors J, Nesic M, Rasmussen MH, Reinert T, Larsen OH, Jaensch C, Løve US, Andersen PV, Kolbro T, Thorlacius-Ussing O, Monti A, Gögenur M, Kildsig J, Bondeven P, Schlesinger NH, Iversen LH, Gotschalck KA, Andersen CL.
Ann Oncol. 2024 Feb;35(2):229-239. doi: 10.1016/j.annonc.2023.11.009. Epub 2023 Nov 21.PMID: 3799287
Cell-Free Urine and Plasma DNA Mutational Analysis Predicts Neoadjuvant Chemotherapy Response and Outcome in Patients with Muscle-Invasive Bladder Cancer.
Christensen E, Nordentoft I, Birkenkamp-Demtröder K, Elbæk SK, Lindskrog SV, Taber A, Andreasen TG, Strandgaard T, Knudsen M, Lamy P, Agerbæk M, Jensen JB, Dyrskjøt L.
Clin Cancer Res. 2023 Apr 14;29(8):1582-1591. doi: 10.1158/1078-0432.CCR-22-3250.PMID: 36780195
Circulating Tumor DNA in Stage III Colorectal Cancer, beyond Minimal Residual Disease Detection, toward Assessment of Adjuvant Therapy Efficacy and Clinical Behavior of Recurrences.
Henriksen TV, Tarazona N, Frydendahl A, Reinert T, Gimeno-Valiente F, Carbonell-Asins JA, Sharma S, Renner D, Hafez D, Roda D, Huerta M, Roselló S, Madsen AH, Løve US, Andersen PV, Thorlacius-Ussing O, Iversen LH, Gotschalck KA, Sethi H, Aleshin A, Cervantes A, Andersen CL.
Clin Cancer Res. 2022 Feb 1;28(3):507-517. doi: 10.1158/1078-0432.CCR-21-2404. Epub 2021 Oct 8.PMID: 34625408
Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma.
Christensen E, Birkenkamp-Demtröder K, Sethi H, Shchegrova S, Salari R, Nordentoft I, Wu HT, Knudsen M, Lamy P, Lindskrog SV, Taber A, Balcioglu M, Vang S, Assaf Z, Sharma S, Tin AS, Srinivasan R, Hafez D, Reinert T, Navarro S, Olson A, Ram R, Dashner S, Rabinowitz M, Billings P, Sigurjonsson S, Andersen CL, Swenerton R, Aleshin A, Zimmermann B, Agerbæk M, Lin CJ, Jensen JB, Dyrskjøt L.
J. Clin Oncol. 2019 Jun 20;37(18):1547-1557. doi: 10.1200/JCO.18.02052. Epub 2019 May 6.PMID: 31059311
Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer.
Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, Knudsen M, Nordentoft I, Wu HT, Tin AS, Heilskov Rasmussen M, Vang S, Shchegrova S, Frydendahl Boll Johansen A, Srinivasan R, Assaf Z, Balcioglu M, Olson A, Dashner S, Hafez D, Navarro S, Goel S, Rabinowitz M, Billings P, Sigurjonsson S, Dyrskjøt L, Swenerton R, Aleshin A, Laurberg S, Husted Madsen A, Kannerup AS, Stribolt K, Palmelund Krag S, Iversen LH, Gotschalck Sunesen K, Lin CJ, Zimmermann BG, Lindbjerg Andersen C.
JAMA Oncol. 2019 Aug 1;5(8):1124-1131. doi: 10.1001/jamaoncol.2019.0528.PMID: 31070691
- Amsterdam UMC
- ELBS Team
- Profile Liquid Biopsy Research
-
Amsterdam UMC


Amsterdam University Medical Centers (Amsterdam UMC) has arisen from the merger of Amsterdam’s two academic hospitals, the Academic Medical Center (AMC) and the VU University Medical Center (VUmc) in 2018. Amsterdam UMC is a leading medical center that combines complex high-quality patient care, state-of-the-art scientific research and top-level education and training of the next generation of health care professionals.
The Amsterdam Vesicle Center is a collaboration between the Department of Biomedical Engineering & Physics and the Laboratory of Experimental Clinical Chemistry of the Amsterdam UMC. By combining biological, chemical, metrological and physical knowledge and expertise, we develop novel technologies to study the presence and biological function of extracellular vesicles (EVs) in health and disease, with the goal to use EVs as clinical biomarkers.
-
-
ELBS Team

Rienk Nieuwland
Principal Investigator
Laboratory of Experimental Clinical Chemistry and Amsterdam Vesicle Center
Meibergdreef 9, 1105 AZ Amsterdam
The Netherlands
Jillian Bracht
Postdoctoral Researcher
Laboratory of Experimental Clinical Chemistry and Amsterdam Vesicle Center
Meibergdreef 9, 1105 AZ Amsterdam
The Netherlands -
-
Profile Liquid Biopsy Research
Cancer types Solid tumors and hematological malignancies. Clinical application Early detection of cancer, monitoring
therapy response, identification of therapy targets and resistance mechanisms.Liquid Biopsy Source Blood, plasma, serum, urine, cerebrospinal fluid, milk, saliva. Technologies available for liquid biopsy Flow cytometry (Apogee A60-Micro, Cytek Northern lights, BD FACS Canto amongst others), Microfluidic resistive pulse sensing (MRPS; nCS1), MRPS plus fluorescence phenotyping (ARC), Size-exclusion chromatography (SEC), Surface plasmon resonance (SPR). Biobank Plasma. Key publications Bracht JWP, Los M, van Eijndhoven MAJ, Bettin B, van der Pol E, Pegtel DM, Nieuwland R. Platelet removal from human blood plasma improves detection of extracellular vesicle-associated miRNA. J Extracell Vesicles. 2023 Feb;12(2):e12302. doi: 10.1002/jev2.12302. PMID: 36788785; PMCID: PMC9929339.
Welsh JA, Arkesteijn GJA, Bremer M, Cimorelli M, Dignat-George F, Giebel B, Görgens A, Hendrix A, Kuiper M, Lacroix R, Lannigan J, van Leeuwen TG, Lozano-Andrés E, Rao S, Robert S, de Rond L, Tang VA, Tertel T, Yan X, Wauben MHM, Nolan JP, Jones JC, Nieuwland R, van der Pol E. A compendium of single extracellular vesicle flow cytometry. J Extracell Vesicles. 2023 Feb;12(2):e12299. doi: 10.1002/jev2.12299. PMID: 36759917; PMCID: PMC9911638.
Clayton A, Boilard E, Buzas EI, Cheng L, Falcón-Perez JM, Gardiner C, Gustafson D, Gualerzi A, Hendrix A, Hoffman A, Jones J, Lässer C, Lawson C, Lenassi M, Nazarenko I, O'Driscoll L, Pink R, Siljander PR, Soekmadji C, Wauben M, Welsh JA, Witwer K, Zheng L, Nieuwland R. Considerations towards a roadmap for collection, handling and storage of blood extracellular vesicles. J Extracell Vesicles. 2019 Aug 1;8(1):1647027. doi: 10.1080/20013078.2019.1647027. PMID: 31489143; PMCID: PMC6711123.
Clayton A, Buschmann D, Byrd JB, Carter DRF, Cheng L, Compton C, Daaboul G, Devitt A, Falcon-Perez JM, Gardiner C, Gustafson D, Harrison P, Helmbrecht C, Hendrix A, Hill A, Hoffman A, Jones JC, Kalluri R, Kang JY, Kirchner B, Lässer C, Lawson C, Lenassi M, Levin C, Llorente A, Martens-Uzunova ES, Möller A, Musante L, Ochiya T, Pink RC, Tahara H, Wauben MHM, Webber JP, Welsh JA, Witwer KW, Yin H, Nieuwland R. Summary of the ISEV workshop on extracellular vesicles as disease biomarkers, held in Birmingham, UK, during December 2017. J Extracell Vesicles. 2018 May 17;7(1):1473707. doi: 10.1080/20013078.2018.1473707. PMID: 31162490; PMCID: PMC5965025.
van der Pol E, Coumans FA, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, Sturk A, van Leeuwen TG, Nieuwland R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. 2014 Jul;12(7):1182-92. doi: 10.1111/jth.12602. Epub 2014 Jun 19. PMID: 24818656.
- Democritus University of Thrace
- ELBS Team
- Profile Liquid Biopsy Research
-
Democritus University of Thrace
 DUTH logo
DUTH logo
 DUTH facilities
DUTH facilities
 DUTH team
DUTH team
We are a hybrid in silico and wet lab translational biomedical research team focusing on:
Biomarker Discovery for Precision Medicine
Aim: to improve the quality of clinical decisions and accelerate medical research to bring solutions for people.
Scientific approach: We apply data- and biology-driven approaches to produce highly performing biosignatures specific for clinically significant disease end-points, in multiple diseases including cancer and diabetes. We employ innovative automated machine learning and text data mining bioinformatic tools to optimise and accelerate biomarker discovery. We also validate models in patient cohorts, focusing on liquid biopsies, to offer mature blood-based solutions readily available for minimally invasive diagnostics anticipated to disrupt clinical management. Novel data-driven drug targets are also identified.
The team is based at the Department of Medicine of the Democritus University of Thrace (med.duth.gr), in close proximity to the University Hospital of Alexandroupolis (pgna.gr), the major health provider of the prefecture of East Macedonia and Thrace (>600,000habitants) in Greece.
The PI Ekaterini Alexiou Chatzaki (PhD) is Professor in Pharmacology at DUTH and Director of the Institute of Agri-Health, Hellenic Mediterranean University Research Centre, Crete, Greece. She leads the team since 2002, with special interests in Epigenetic/Pharmacoepigenetics. The team currently consists of 5 post-docs and numerous PhD and post-graduate Biologists.
-
-
ELBS Team

Prof. Ekaterini Alexiou Chatzaki
Lab. Pharmacology, Med-DUTH, GR
ORCID ID: 0000-0002-5832-4257
ResearchGate: Ekaterini Chatzaki
linkedin: Ekaterini Chatzaki, Chatzaki teamDr. Theodosis Theodosiou
Lab. Pharmacology, Med-DUTH, GR
Career Stage: Category C Recognized researcher
ORCID ID: 0000-0002-6151-1803Dr. Makrina Karaglani
Lab. Pharmacology, Med-DUTH, GR
Career Stage: post-doc, Category C Recognized researcher
ORCID ID: 0000-0002-9853-3571Dr. Maria Panagopoulou Pantazi
Lab. Pharmacology, Med-DUTH, GR
Career Stage: post-doc, Category C Recognized researcher
ORCID ID: 0000-0002-7107-4774Dr. Maria Christina Cheimonidou
Lab. Pharmacology, Med-DUTH, GR
Career Stage: post-doc, Category C Recognized researcher
-
-
Profile Liquid Biopsy Research
Cancer types Breast Cancer, Pancreatic Cancer.
Other diseases: diabetes, prediabetes, schizophrenia.Clinical application Diagnosis/prognosis/disease monitoring/pharmacotherapy response, screening. Liquid Biopsy Source Blood, plasma. Technologies available for liquid biopsy Digital droplet PCR, LightCycler 480, Flow cytometry, Immunofluoresence microscopy, Raman spectrophotometry, cell culture. Biobank Plasma samples and clinical information collected from patient cohorts from above mentioned diseases. Key publications Circulating cell-free DNA in breast cancer: size profiling, levels, and methylation patterns lead to prognostic and predictive classifiers. Panagopoulou M, Karaglani M, Balgkouranidou I, Biziota E, Koukaki T, Karamitrousis E, Nena E, Tsamardinos I, Kolios G, Lianidou E, Kakolyris S, Chatzaki E.Oncogene. 2019 May;38(18):3387-3401. doi: 10.1038/s41388-018-0660-y.
Liquid Biopsy in Type 2 Diabetes Mellitus Management: Building Specific Biosignatures via Machine Learning. Karaglani M, Panagopoulou M, Cheimonidi C, Tsamardinos I, Maltezos E, Papanas N, Papazoglou D, Mastorakos G, Chatzaki E. J Clin Med. 2022 Feb 17;11(4):1045. doi: 10.3390/jcm11041045.
Deciphering the Methylation Landscape in Breast Cancer: Diagnostic and Prognostic Biosignatures through Automated Machine Learning. Panagopoulou M, Karaglani M, Manolopoulos VG, Iliopoulos I, Tsamardinos I, Chatzaki E. Cancers (Basel). 2021 Apr 2;13(7):1677. doi: 10.3390/cancers13071677.
Just Add Data: automated predictive modeling for knowledge discovery and feature selection. Tsamardinos I, Charonyktakis P, Papoutsoglou G, Borboudakis G, Lakiotaki K, Zenklusen JC, Juhl H, Chatzaki E, Lagani V. NPJ Precis Oncol. 2022 Jun 16;6(1):38. doi: 10.1038/s41698-022-00274-8.
Automated machine learning optimizes and accelerates predictive modeling from COVID-19 high throughput datasets. Papoutsoglou G, Karaglani M, Lagani V, Thomson N, Røe OD, Tsamardinos I, Chatzaki E. Sci Rep. 2021 Jul 23;11(1):15107. doi: 10.1038/s41598-021-94501-0.
- Erasmus MC
- ELBS Team
- Profile Liquid Biopsy Research
-
Erasmus MC


The department of Medical Oncology is one of the departments of the Erasmus MC, the largest university medical center in the Netherlands with more than 14,000 employees. Within the Erasmus MC, the department is part of the Erasmus MC Cancer Institute in which all departments involved in cancer care, education and/or research work together. In addition to providing care to patients with solid malignancies and training of oncology students and residents, the department of medical oncology focuses strongly on clinical and translational research. Researchers from the department have had coordinating roles in studies impacting the world-wide clinical treatment standards in several tumor types including testicular cancer, prostate cancer, bladder cancer, soft tissue sarcomas, and esophageal cancer. Apart from the more than 150 ongoing clinical studies, both pharma- and investigator-initiated, one of the research areas of interest of the department is the development and testing of minimally invasive techniques to capture the molecular characteristics of tumor cells important for treatment decision making. Studies have been done on the robustness of different technologies for liquid biopsies and numerous studies have been done to assess the clinical value of liquid biopsies including in total more than 3,500 patients.
-
-
ELBS Team

Bianca Mostert, MD, PhD
Medical Oncologist and Head of Department Medical Oncology
Dr. Molewaterplein 40
3015 GD Rotterdam NetherlandsProf. dr. John Martens, PhD
Head of lab Translational Cancer Genomics and Proteomics, Department of Medical Oncology
Dr. Molewaterplein 40
3015 GD Rotterdam NetherlandsDr. Jaco Kraan, PhD
Lab Translational Cancer Genomics and Proteomics, Department of Medical Oncology
Dr. Molewaterplein 40
3015 GD Rotterdam Netherlands -
-
Profile Liquid Biopsy Research
Cancer types Breast cancer, prostate cancer, colorectal cancer, bladder cancer, melanoma Clinical application Prognostic and predictive value of liquid biopsies, value as early marker of response. Liquid Biobsy Source Blood, plasma, liquor Technologies available for liquid biopsy CellSearch, Cytotrack, VyCAP, several NGS platforms Biobank Solid tumors (plasma) Key publications Priestley P, Baber J, Lolkema MP, Steeghs N, de Bruijn E, Shale C, Duyvesteyn K, Haidari S, van Hoeck A, Onstenk W, Roepman P, Voda M, Bloemendal HJ, Tjan-Heijnen VCG, van Herpen CML, Labots M, Witteveen PO, Smit EF, Sleijfer S, Voest EE, Cuppen E. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature. 2019 Nov; 575(7781):210-216. doi: 10.1038/s41586-019-1689-y
Angus L, Smid M, Wilting SM, van Riet J, Van Hoeck A, Nguyen L, Nik-Zainal S, Steenbruggen TG, Tjan-Heijnen VCG, Labots M, van Riel JMGH, Bloemendal HJ, Steeghs N, Lolkema MP, Voest EE, van de Werken HJG, Jager A, Cuppen E, Sleijfer S, Martens JWM. The genomic landscape of metastatic breast cancer highlights changes in mutation and signature frequencies. Nat Genet. 2019 Oct;51(10):1450-1458. doi: 10.1038/s41588-019-0507-7
Onstenk W, Sieuwerts AM, Kraan J, Van M, Nieuweboer AJ, Mathijssen RH, Hamberg P, Meulenbeld HJ, De Laere B, Dirix LY, van Soest RJ, Lolkema MP, Martens JW, van Weerden WM, Jenster GW, Foekens JA, de Wit R, Sleijfer S. Efficacy of Cabazitaxel in Castration-resistant Prostate Cancer Is Independent of the Presence of AR-V7 in Circulating Tumor Cells. Eur Urol. 2015 Dec;68(6):939-45. doi: 10.1016/j.eururo.2015.07.007
van Dessel LF, Beije N, Helmijr JC, Vitale SR, Kraan J, Look MP, de Wit R, Sleijfer S, Jansen MP, Martens JW, Lolkema MP. Application of circulating tumor DNA in prospective clinical oncology trials - standardization of preanalytical conditions. Mol Oncol. 2017 Mar;11(3):295-304. doi: 10.1002/1878-0261.12037
Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, Martens JW, Gratama JW, Sleijfer S, Foekens JA Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. .J Natl Cancer Inst. 2009 Jan 7;101(1):61-6. doi: 10.1093/jnci/djn419
- Hahn-Schickard
- ELBS team
- Profile Liquid Biopsy Research
-
Hahn-Schickard


Lab-on-a-chip – from the initial idea to the final product. Hahn-Schickard stands for client- and industry-oriented, application-driven research, development, and production with microsystems. With a total of 250 employees at three sites in the Southwest of Germany, we develop innovative products and technologies in research fields with a strong future impact such as healthcare, internet of things, information and communication technologies, sustainable mobility, as well as environmental and natural resources. At the site in Freiburg, the R+D service provider focusses on automation solutions for diagnostics. Fields of research are the automation of Liquid Biopsy workflows and sample preparation for next generation sequencing. With an in-house pilot line, Hahn-Schickard is able to support the product visions of its customers effectively – from the design stage to serial production.
-
-
ELBS team

Dr. Tobias Hutzenlaub
Group leader Microfluidic Platforms
Georges-Koehler-Allee 103
79110 Freiburg
Germany
Tobias.Hutzenlaub@Hahn-Schickard.de

Peter Jülg
Group leader Microfluidic Platforms
Georges-Koehler-Allee 103
79110 Freiburg
Germany
Peter.Juelg@Hahn-Schickard.de
-
-
Profile Liquid Biopsy Research
Cancer types Colorectal cancer, melanoma, acute lymphoblastic leukemia Clinical application Monitoring of therapy response, early detection of relapse Liquid Biopsy source blood, bone marrow Technologies available for Liquid Biopsy Centrifugal microfluidic platform consisting of processing device (ready for certification in 2020) and application specific microfluidic chips for ctDNA extraction, qPCR, dPCR and sample preparation for next generation sequencing. Key publications O. Strohmeier, M. Keller, F. Schwemmer, S. Zehnle, D. Mark, F. von Stetten, R. Zengerle and N. Paust, Centrifugal microfluidic platforms: advanced unit operations and applications, Chem. Soc. Rev., 2015, 44, 6187
P. Juelg, M. Specht, E. Kipf, M. Lehnert, C. Eckert, M. Keller, T. Hutzenlaub, F. von Stetten, R. Zengerle and N. Paust, Automated serial dilutions for high-dynamic-range assays enabled by fill-level-coupled valving in centrifugal microfluidics, Lab Chip, 2019, 19, 2205
J.F. Hess, T.A. Kohl, M. Kotrová, K Rönsch, T. Paprotka, V. Mohr, T. Hutzenlaub, M. Brüggemann, R. Zengerle, S. Niemann, N. Paust, Library preparation for next generation sequencing: A review of automation strategies, Biotechnology Advances 2020, in press
J. Friedrich Hess, M. Kotrová, S. Calabrese, T. Hutzenlaub, R. Zengerle, M. Brueggemann, N. Paust, Automation of amplicon-based library preparation for next generation sequencing by centrifugal microfluidics, 2020, under review
- IRCCS Regina Elena Cancer Research Institute
- ELBS Team
- Profile Liquid Biopsy Research
-
IRCCS Regina Elena Cancer Research Institute

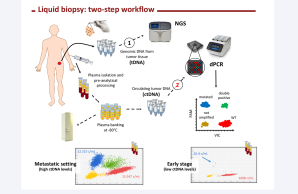
The Regina Elena Institute (IRE) is one of the two twin Institutes forming Istituti Fisioterapici Ospitalieri (IFO). Founded in 1934, IRE is possibly the oldest Cancer Research Institute in Europe. It includes research laboratories, clinical research services and hospital assistance staffed with >1000 units of personnel. IRE is a member of the ‘Union International Contre le Cancer’ (U.I.C.C.), of the European Organization of Cancer Institutes (O.E.C.I.), of the European Organization for Research and Treatment of Cancer (E.O.R.T.C. - Early ClinicaI Trial Group), and is referral center of the World Health Organization (WHO). Since 2015 IRE has been accredited as O.E.C.I. Comprehensive Cancer Center. IRE actively investigates mechanisms of neoplastic transformation and progression using transcriptomics, proteomics, molecular diagnosis, predictive oncology, and aims at developing targeted therapeutics. IRE features a fully certified BioBank (BB-IRE) for tissue and liquid biopsy. Since 2016 we perform Liquid Biopsy in the standard of care setting (EGFR, Lung cancer). The research laboratories are organized in a large 2500 square meter open space including central facilities. Pre-clinical and co-clinical (PDx) animal studies are ongoing, including a focus on liquid biopsy. IRE has participated and/or is currently involved in several H2020 EU project on liquid biopsy including ULTRAPLACAD (www.ultraplacad.eu), AiPBAND (www.aipband-itn.eu), and OncNGS (http://oncngs.eu).
-
-
ELBS Team

Prof. Gennaro Ciliberto
Scientific Director
IRCCS Regina Elena Cancer Research Institute
Via Elio Chianesi 53
00144 Rome
Italy
gennaro.ciliberto@ifo.gov.it

Dr. Patrizio Giacomini
Principal investigator
Oncogenomics and Epigenetics
Via Elio Chianesi 53
00144 Rome
Italy
patrizio.giacomini@ifo.gov.it
-
-
Profile Liquid Biopsy Research
Cancer types Solid tumors (e.g. lung, breast, Gl-tract, melanoma, bladder, brain tumors, H&N cancer) and hematological malignancies (DLBCL). Rare tumors (sarcoma and thyroid). Clinical application ctDNA to assign target therapy in the context of the Standard of Care (SoC) setting, real-life clinical trials, and the activities of our Molecular Tumor Board (IRE-MTB). ctDNA to reveal actionable resistance to treatment. ctDNA in the peri-surgical period (Colorectal). Circulating fusion transcripts in rare tumors (sarcoma). miRNA signatures for diagnosis and treatment (melanoma, bladder, DLBCL, Colorectal). Liquid Biopsy and Radiogenomics. Liquid Biobsy Source Plasma, Cerebrospinal Fluid (CSF), urine, pleural effusions Technologies available for liquid biopsy Targeted and custom NGS panels; miRNome analysis. Thermofisher S5 and IonChef, Illumina MiSeq and NextSeq; nanostring and pyrosequencing equipment; conventional (Cobas z480 Roche) and digital (Thermofisher QuantStudio 3D) PCR (dPCR); Hamilton Robotic Station, DEParray Menarini; Affymetrix Station Biobank Tissue (BBIRE-T) and body fluids (BBIRE-L) BioBanking; >20.000 and >50.000 samples stored, respectively Key publications Regazzo G, Terrenato I, Spagnuolo M, Carosi M, Cognetti G, Cicchillitti L, Sperati F, Villani V, Carapella C, Piaggio G, Pelosi A, Rizzo MG.A restricted signature of serum miRNAs distinguishes glioblastoma from lower grade gliomas. J Exp Clin Cancer Res 2016 Jul 30;35(1):124. doi: 10.1186/s13046-016-0393-07. 2. Sinibaldi, A., C.
Sampaoli, N. Danz, P. Munzert, L. Sibilio, F. Sonntag, A. Occhicone, E. Falvo, E. Tremante, P. Giacomini, and F. Michelotti. Detection of soluble ERBB2 in breast cancer cell lysates using a combined label-free/fluorescence platform based on Bloch surface waves. Biosens Bioelectron 2017 92: 125-130.
Marchesi F, Regazzo G, Palombi F, Terrenato I, Sacconi A, Spagnuolo M, Donzelli S, Marino M, Ercolani C, Di Benedetto A, Blandino G, Ciliberto G, Mengarelli A, Rizzo MG. Serum miR-22 as potential non-invasive predictor of poor clinical outcome in newly diagnosed, uniformly treated patients with diffuse large B-cell lymphoma: an explorative pilot study. J Exp Clin Cancer Res. 2018 May 2;37(1):95. doi: 10.1186/s13046-018-0768-5.
Allegretti, M., B. Casini, C. Mandoj, S. Benini, L. Alberti, M. Novello, E. Melucci, L. Conti, R. Covello, E. Pescarmona, G. M. Milano, A. Annovazzi, V. Anelli, V. Ferraresi, R. Biagini, and P. Giacomini. Precision diagnostics of Ewing's sarcoma by liquid biopsy: circulating EWS-FLI1 fusion transcripts. Ther Adv Med Oncol 2018 10: 1758835918774337.
Allegretti, M., G. Cottone, F. Carboni, E. Cotroneo, B. Casini, E. Giordani, C. A. Amoreo, S. Buglioni, M. Diodoro, E. Pescarmona, S. Zazza, O. Federici, M. Zeuli, L. Conti, G. Cigliana, F. Fiorentino, M. Valle, P. Giacomini, and F. Spinella. Cross-sectional analysis of circulating tumor DNA in primary colorectal cancer at surgery and during post-surgery follow-up by liquid biopsy. Journal of Experimental & Clinical Cancer Research 2020 CR 39: 69.
- Iwate Medical University
- ELBS Team
- Profile Liquid Biopsy Research
-
Iwate Medical University


Approximately 2 hours by Japanese Shinkansen bullet train from Tokyo, the Iwate Medical University Institute for Biomedical Sciences was established in 2011 as a part of the new Iwate Medical University Yahaba Campus. The Yahaba Campus holds three professional schools, the university hospital system, and the research institute, which is one of the most cutting-edge medical research institutions in northern Japan. The Division of Biomedical Research & Development was funded in 2016 at the institute and focuses on the clinical implementation of circulating tumor DNA (ctDNA) for cancer diagnosis under a tight association with the University Hospital.
Our ultimate goal is to establish a real-time, quantitative, and longitudinal monitoring system for post-treatment cancer patients using ctDNA. Digital PCR (dPCR) is considered to be one of the most suitable techniques for repeatedly quantifying ctDNA in an affordable manner. However, a dPCR assay requires an individually designed primer/probe set that is dependent on each somatic mutation of the patient. To circumvent that issue, we developed a pre-made dPCR primer/probe library that contains more than 1,000 target mutations. The patent rights for this dPCR primer/probe library have been transferred to an Iwate Medical University spinout venture, Quantdetect, Inc. (Tokyo, Japan). The dPCR primer/probe library is now used for post cancer-treatment surveillance, namely the OTS-Assay (OTS, off-the-shelf), at the Iwate Medical University Hospital.
-
-
ELBS Team

Satoshi Nishizuka, M.D., Ph.D.
Professor and Principal Investigator
Division of Biomedical Research & Development
Iwate Medical University
Institute for Biomedical Sciences
1-1-1 Idaidori W-Research Bldg rm101
028-3694 Yahaba
Japan
Takeshi Iwaya, M.D., Ph.D.
Professor
Department of Clinical Oncology
Iwate Medical University School of Medicine
1-1-1 Idaidori W-Research Bldg rm101
028-3694 Yahaba
Japan
Akiko Yahima-Abo, M.D., Ph.D.
Assistant Professor
Division of Biomedical Research & Development
Iwate Medical University
Institute for Biomedical Sciences1-1-1 Idaidori W-Research Bldg rm101
028-3694 Yahaba
Japan
Hayato Hiraki, Ph.D.
Lecturer
Division of Biomedical Research & Development
Iwate Medical University
Institute for Biomedical Sciences1-1-1 Idaidori W-Research Bldg rm101
028-3694 Yahaba
Japan -
-
Profile Liquid Biopsy Research
Cancer types Any. Gastrointestinal, lung, bladder, etc. Clinical application Early relapse prediction; treatment efficacy evaluation; no relapse corroboration. Liquid Biopsy Source Plasma, urine, bone marrow. Technologies available for liquid biopsy Digital PCR primer/probe library (OTS-Probes); multiple digital PCR platforms. Biobank Solid tumors; urine; bone marrow Key publications Fujisawa, R., Iwaya, T., Endo, F., Idogawa, M., Sasaki, N., Hiraki, H., Tange, S., Hirano, T., Koizumi, Y., Abe, M., Takahashi, T., Yaegashi, M., Akiyama, Y., Masuda, M., Sasaki, A., Takahashi, F., Sasaki, Y., Tokino, T. & Nishizuka, S.S. Early dynamics of circulating tumor DNA predict chemotherapy responses for patients with esophageal cancer. Carcinogenesis 42, 1239-1249 (2021).
Iwaya, T., Endo, F., Takahashi, F., Tokino, T., Sasaki, Y. & Nishizuka, S.S. Frequent Tumor Burden Monitoring of Esophageal Squamous Cell Carcinoma With Circulating Tumor DNA Using Individually Designed Digital Polymerase Chain Reaction. Gastroenterology 160, 463-465 e464 (2021).
Yaegashi, M., Iwaya, T., Sasaki, N., Fujita, M., Ju, Z., Siwak, D., Hachiya, T., Sato, K., Endo, F., Kimura, T., Otsuka, K., Sugimoto, R., Sugai, T., Liotta, L., Lu, Y., Mills, G.B., Nakagawa, H. & Nishizuka, S.S. Frequent post-operative monitoring of colorectal cancer using individualised ctDNA validated by multiregional molecular profiling. Br J Cancer 124, 1556-1565 (2021).
Sasaki, N., Iwaya, T., Chiba, T., Fujita, M., Ju, Z., Endo, F., Yaegashi, M., Hachiya, T., Sugimoto, R., Sugai, T., Siwak, D.R., Liotta, L.A., Lu, Y., Mills, G.B., Nakagawa, H. & Nishizuka, S.S. Analysis of mutational and proteomic heterogeneity of gastric cancer suggests an effective pipeline to monitor post-treatment tumor burden using circulating tumor DNA. PLoS One 15, e0239966 (2020).
Nishizuka, S.S., Sato, K.A. & Hachiya, T. A Pipeline for ctDNA Detection Following Primary Tumor Profiling Using a Cancer-Related Gene Sequencing Panel. Methods Mol Biol 1908, 229-241 (2019).
- Jessa Hospital Hasselt
- ELBS Team
- Profile Liquid Biopsy Research
-
Jessa Hospital Hasselt

The Jessa Hospital is a regional reference hospital with 1000 beds and more than 3000 employees. It offers innovative and highly qualitative medicine and patient care in collaboration with several partnering hospitals. Next to the classical medical specialties the Jessa hospital also offers a number of highly specialized top clinical services that requires advanced equipment. The Laboratory for Molecular Diagnostics is amongst the 9 Belgian NGS networks and one of the first in Belgium to diagnostically implement NGS for the genomic profiling of cancer patients. Currently, we perform targeted small as well as comprehensive NGS gene panels for the somatic screening of solid and hematological tumors. The lab is leading the BALLETT study (NCT05058937) to assess the added clinical value of comprehensive genomic profiling in routine care, with discussion of the patient’s results in a national Molecular Tumor Board. The lab has broad expertise on liquid biopsy analysis as it performs NIPT for more than 7 years. Recently, NGS on cfDNA was also validated for the routine screening of somatic hotspot mutations, mainly in NSCLC and breast cancer patients. On short term we aim to significantly expand the gene panel allowing for broad screening of multiple aberrations and biomarkers.
-
-
ELBS Team

Brigitte Maes, MD, PhD
Clinical Pathologist
Head of Laboratory for Molecular Diagnostics
-
-
Profile Liquid Biopsy Research
Cancer types Mainly NSCLC and breast cancer. Clinical application Primary analysis, follow-up and resistance. Liquid Biopsy Source Plasma Technologies available for liquid biopsy cfDNA QiaSeq targeted custom panel; aim to switch to a much larger panel. Biobank Plasma, cfDNA Key publications Liquid biopsy-related paper
Vandekerckhove O, Cuppens K, Pat K, Du Pont B, Froyen G, Maes B. Liquid Biopsy in Early-Stage Lung Cancer: Current and Future Clinical Applications. Cancers 2023; 15, 2702.
Other papers
Froyen G, Geerdens E, Berden S, Cruys B, Maes B. Diagnostic Validation of a Comprehensive Targeted Panel for Broad Mutational and Biomarker Analysis in Solid Tumors. Cancers 2022;14(10):2457. PMID: 35626061
Thouvenin J, Van Marcke C, Decoster L, et al. PRECISION: the Belgian molecular profiling program of metastatic cancer for clinical decision and treatment assignment. ESMO Open. 2022;7(4):100524. PMID: 35970014
Froyen G, Le Mercier M, Lierman E, et al. Standardization of Somatic Variant Classifications in Solid and Haematological Tumours by a Two-Level Approach of Biological and Clinical Classes: An Initiative of the Belgian ComPerMed Expert Panel. Cancers 2019;11(12):2030. PMID: 31888289
Froyen G, Maes B. Clinical Validation of Targeted Solid Tumor Profiling. Methods Mol Biol. 2019;1908:73-87. PMID: 30649722
- KU/UZ Leuven
- ELBS Team
- Profile Liquid Biopsy Research
-
KU/UZ Leuven

The Center for Human Genetics (CME) at the University of Leuven, conducts cutting edge research within the areas of human genetics and genomics in hereditary diseases and cancer. We put our clinical services and scientific knowhow at the disposal of our patients, by offering state of the art diagnostic and counseling facilities, and potential clinical strategies.
The laboratory for Cytogenetics and Genome Research spearheads technologies to map the causes and mechanisms underlying rare developmental disorders, improve diagnosis of rare diseases and prenatal and preimplantation genetic diagnosis to avoid transmission of disease alleles. The lab has over 15 years of experience in liquid biopsies, and was among the first to implement non-invasive prenatal testing (NIPT) in a clinical setting in Europe. The team demonstrated that NIPT profiling enables detection of incipient tumours, underpinning the value of cfDNA as a cancer screening tool. The latest in-house developed machine-learning-based pipeline offers a cost-effective screening tool for pan-cancer detection and typing from shallow whole-genome sequencing data.
The Cancer and pregnancy interdisciplinary research in the Gynaecological Oncology laboratory focuses on the diagnosis and treatment of pregnant cancer patients and monitoring of newborns that were prenatally exposed to chemotherapy. In this framework, we are performing in vitro, in vivo, ex vivo and clinical studies. We also have a special interest in the use of cfDNA for detecting cancer during pregnancy.
-
-
ELBS Team

Prof. Joris Vermeesch
Head of the Laboratory for Cytogenetics and Genome Research
Herestraat 49, 3000 Leuven, Belgium

Liesbeth Lenaerts
Research Manager in the Gynaecological Oncology Laboratory
Herestraat 49, 3000 Leuven, Belgium

Tatjana Jatsenko
Postdoctoral Researcher in the Lab for Cytogenetics and Genome Research
Herestraat 49, 3000 Leuven, Belgium

Tom Broeckx
Coordinator Genomics Core Leuven
Herestraat 49, 3000 Leuven, Belgium
-
-
Profile Liquid Biopsy Research
Cancer types Solid (breast ovarian, cervical, colorectal, lung) and hematological (Hodgkin’s lymphoma, multiple myeloma, diffuse large B cell lymphoma) tumors Clinical application early detection of cancer, cancer in pregnancy Liquid Biopsy Source blood plasma, urine Technologies available for liquid biopsy In house developed pipeline for CNA detection (GIPSeq); machine-learning-based cfDNA profiling pipeline for (pan)cancer detection and typing (GIPXplore); enzymatic-conversion-based methylation analyses: in-house deconvolution algorithms and single-read methylation analysis (under development). Scalable cloud-based platform to improve global accessibility and broad implementation of cfDNA analytical tools (under development). Biobank cell-free DNA (cfDNA) Key publications Lenaerts L, Vandenberghe P, Brison N, et al. Genomewide copy number alteration screening of circulating plasma DNA: potential for the detection of incipient tumors. Ann Oncol. 2019;30(1):85-95. doi:10.1093/annonc/mdy476
Amant F, Verheecke M, Wlodarska I, et al. Presymptomatic Identification of Cancers in Pregnant Women During Noninvasive Prenatal Testing. JAMA Oncol. 2015;1(6):814-819. doi:10.1001/jamaoncol.2015.1883
Bayindir B, Dehaspe L, Brison N, et al. Noninvasive prenatal testing using a novel analysis pipeline to screen for all autosomal fetal aneuploidies improves pregnancy management. Eur J Hum Genet. 2015;23(10):1286-1293. doi:10.1038/ejhg.2014.282
Vandenberghe P, Wlodarska I, Tousseyn T, et al. Non-invasive detection of genomic imbalances in Hodgkin/Reed-Sternberg cells in early and
advanced stage Hodgkin's lymphoma by sequencing of circulating cell-free DNA: a technical proof-of-principle study. Lancet Haematol. 2015;2(2):e55-e65. doi:10.1016/S2352-3026(14)00039-8
Lenaerts L, Brison N, Maggen C, et al. Comprehensive genome-wide analysis of routine non-invasive test data allows cancer prediction: A single-center retrospective analysis of over 85,000 pregnancies. EClinicalMedicine. 2021;35:100856. Published 2021 May 13. doi:10.1016/j.eclinm.2021.100856
Che H., Jatsenko T., Lenaerts L., et al. Pan-cancer detection and typing by mining patterns in large genome-wide cell-free DNA sequencing datasets. medRxiv 2022.02.16.22268780; doi: https://doi.org/10.1101/2022.02.16.22268780 (under revision)
- Kyushu University Beppu Hospital
- ELBS Team
- Profile Liquid Biopsy Research
-
Kyushu University Beppu Hospital
 Kyushu University Beppu Hosptial
Kyushu University Beppu Hosptial
Kyushu University Beppu Hospital was established in 1931 as a branch of the main hospital of Kyushu University in Fukuoka, Japan. We have four medical departments, such as surgery, internal medicine, orthopedics and the radiation in Beppu city. In our department, we have five senior surgeons for operations of 200 digestive system cancers and 100 breast cancers per year and are mentors who orchestrate a dozen graduate students as mentee for research works to disclose the bona-fide truth to eradicate cancers.
In our department of surgery, we organize a lab for basic and translational research work. At first, we are elucidating the cancer evolution to foster the intratumor heterogeneity (ITH) which have attracted increasing attention in the cancer research field because ITH presumably contributes to the therapeutic and diagnostic difficulties of cancer. We applied the recent technological innovation conducting the multiregional sequencing approach, which has been popularly used to understand ITH (Uchi R, PLoS Genet 2016, Saito T. Nat Commun 2018, Yokoyama A., Nature 2019).
The second, we have been enthusiastically identifying cancer specific transcriptomes, non-coding genes, genomic alterations and epigenomic aberrations in the body fluid as the "liquid biopsy" system for early diagnosis of postoperative recurrence, concealed cancers at subclinical level in primary tumors and prediction of the susceptibility to any treatments.
Anyway, it is a great honor for us to join the ELBS meeting, I would appreciate it for the chairperson Prof. Pantel and who may concern.
-
-
ELBS Team

Prof. Koshi Mimori
4546 Tsurumihara,
Beppu 874-0838,
Japan
kmimori@beppu.kyushu-u.ac.jp

Takaaki Masuda
Lecture
4546 Tsurumihara
874-0838 Beppu
Japan
takaakimas@yahoo.co.jp
-
-
Profile Liquid Biopsy Research
Cancer types Digestive system cancers, and breast cancers Clinical application Early detection of recurrence and prediction of the susceptibility to treatment Liquid Biopsy Source Plasma, serum from cancer patients Technologies available for liquid biopsy Target re-sequencing, ddPCR Key publications Sugimachi K, Sakimura S, Kuramitsu S, Hirata H, Niida A, Iguchi T, Eguchi H, Masuda T, Morita M, Toh Y, Maehara Y, Suzuki Y, Mimori K. Serial mutational tracking in surgically resected locally advanced colorectal cancer with neoadjuvant chemotherapy. Br J Cancer. 119(4): 419-23. 2018
Ueda M, Iguchi T, Masuda T, Nakahara Y, Hirata H, Uchi R, Niida A, Momose K, Sakimura S, Chiba K, Eguchi H, Ito S, Sugimachi K, Yamasaki M, Suzuki Y, Miyano S, Doki Y, Mori M, Mimori K. Oncotarget. 7(38): 62280-91. 2016
Iwaya T, Fukagawa T, Suzuki Y, Takahashi Y, Sawada G, Ishibashi M, Kurashige J, Sudo T, Tanaka F, Shibata K, Endo F, Katagiri H, Ishida K, Kume K, Nishizuka S, Iinuma H, Wakabayashi G, Mori M, Sasako M, Mimori K. Contrasting expression patterns of histone mRNA and microRNA 760 in patients with gastric cancer. Clin Cancer Res. 19(23):6438-49. 2013
Mimori K, Fukagawa T, Kosaka Y, Kita Y, Ishikawa K, Etoh T, Iinuma H, Sasako M, Mori M. Hematogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor-1. Clin Cancer Res. 14(9):2609-16. 2008.
Yokobori T, Iinuma H, Shimamura T, Imoto S, Sugimachi K, Ishii H, Iwatsuki M, Ota D, Ohkuma M, Iwaya T, Nishida N, Kogo R, Sudo T, Tanaka F, Shibata K, Toh H, Sato T, Barnard GF, Fukagawa T, Yamamoto S, Nakanishi H, Sasaki S, Miyano S, Watanabe T, Kuwano H, Mimori K, Pantel K, Mori M. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Res. 73(7):2059-69. 2013
- Leiden University Medical Center (LUMC)
- ELBS Team
- Profile Liquid Biopsy Research
-
Leiden University Medical Center (LUMC)


The Leiden University Medical Center (LUMC) in Leiden, The Netherlands, strongly underpins the idea that ‘Science is the driving force behind innovative healthcare’. Outcomes of top level fundamental, translational and clinical research by LUMC researchers form a strong basis for innovative and qualitative healthcare on a national, European and international level.
A real game changer was the development of the Dutch national research agenda (NWA) in 2015. The agenda reveals the complexity of the issues challenging Dutch society today. These issues are clustered in so called routes. LUMC is involved in many of these routes and contributes this way to solving important societal challenges.
The department of Surgery LUMC focusses on cancer, trauma, transplant and complex vascular surgery, each with an extensive research program. We strive to better understand and detect diseases and to develop and apply new treatment techniques. Surgery LUMC has a data center, tissue banks and laboratory facilities at the LUMC. With these advanced resources, we conduct research at an internationally recognized level.
Over the last years our research group build a large track record in the field of biomarkers for prognosis and therapy. The tumour-stroma ratio was through the years developed to a highly accepted biomarker for the adequate selection of patients for adjuvant therapy. Biomarkers for the early detection of cancer in blood using mass spectrometry on serum samples, patients with cancer can be identified from healthy controls. Combining the knowledge of the tumor microenvironment and biomarkers in serum had led to the development of a liquid biopsy panel for tailored treatment and monitoring of patients with epithelial cancer types.
The department of Clinical Chemistry and Laboratory Medicine at the LUMC houses the core facility for routine measurements of patient body fluids at 24-7 basis ( Film Clinical Chemistry Lab LUMC ). Within this department a group of scientists works on assay development with focus on implementing quantitative protein mass spectrometry as a lab-developed test.
-
-
ELBS Team

Dr. Wilma Mesker
Associate professor and head of the laboratory of Surgery
Department of Surgery, LUMC
Albinusdreef 2
2333 ZA Leiden
The Netherlands
Drs. Layla Andour
PhD candidate
Department of Surgery, LUMC
Albinusdreef 2
2333 ZA Leiden
The Netherlands
Dr. Yuri van der Burgts
Associate professor
Department of Clinical Chemistry and Laboratory Medicine, LUMC
Albinusdreef 2
2333 ZA Leiden
The Netherlands
Prof Dr. Christa Cobbaert
Head of the department of Clinical Chemistry and Laboratory Medicine, LUMC
Albinusdreef 2
2333 ZA Leiden
The Netherlands -
-
Profile Liquid Biopsy Research
Cancer types Clinical application Liquid Biopsy Source Technologies available for liquid biopsy Biobank Key publications Development of Tier 2 LC-MRM-MS protein quantification methods for liquid biopsies. Diederiks N, Ravensbergen CJ, Treep M, van Wezel M, Kuruc M, Renee Ruhaak L, Tollenaar RAEM, Cobbaert CM, van der Burgt YEM, Mesker WE.J Mass Spectrom Adv Clin Lab. 2022 Dec 23;27:49-55. doi: 10.1016/j.jmsacl.2022.12.007.
Longitudinal Serum Protein Analysis of Women with a High Risk of Developing Breast Cancer Reveals Large Interpatient Versus Small Intrapatient Variations: First Results from the TESTBREAST Study. Hagenaars SC, Dekker LJM, Ravesteijn B, van Vlierberghe RLP, Romijn FPHTM, Verhoeff L, Witkamp AJ, Schenk KE, Keymeulen KBIM, Menke-Pluijmers MBE, Dassen AE, Kortmann BA, de Vries J, Rutgers EJT, van der Burgt YEM, Meershoek-Klein Kranenbarg E, Cobbaert CM, Luider TM, Mesker WE, Tollenaar RAEM.Int J Mol Sci. 2022 Oct 17;23(20):12399. doi: 10.3390/ijms232012399.
Longitudinal changes of serum protein N-Glycan levels for earlier detection of pancreatic cancer in high-risk individuals. Levink IJM, Klatte DCF, Hanna-Sawires RG, Vreeker GCM, Ibrahim IS, van der Burgt YEM, Overbeek KA, Koopmann BDM, Cahen DL, Fuhler GM, Wuhrer M, Bonsing BA, Tollenaar RAEM, Vleggaar FP, Vasen HFA, van Leerdam ME, Bruno MJ, Mesker WE.Pancreatology. 2022 May;22(4):497-506. doi: 10.1016/j.pan.2022.03.021.
The Stroma Liquid Biopsy Panel Contains a Stromal-Epithelial Gene Signature Ratio That Is Associated with the Histologic Tumor-Stroma Ratio and Predicts Survival in Colon Cancer. Ravensbergen CJ, Kuruc M, Polack M, Crobach S, Putter H, Gelderblom H, Roy D, Tollenaar RAEM, Mesker WE. Cancers (Basel). 2021 Dec 29;14(1):163. doi: 10.3390/cancers14010163.
- MGZ – Medical Genetics Center
- ELBS Team
- Liquid Biopsy Profile
-
MGZ – Medical Genetics Center

Besides the molecular diagnostics of germline genetic variants causal for hereditary diseases, we also focus on the detection of somatic variants, found in cancer and mosaic diseases.
Recent developments include several diagnostic tests for liquid biopsy analysis to support treatment decisions, detection of residual disease, disease monitoring, and even cancer screening. With the use of targeted hotspot assays and Duplex sequencing, we offer the sensitive and specific detection of clinically actionable variants present in lowest variant allele frequencies (VAFs) in plasma. In addition, we have developed a whole-genome sequencing (WGS) approach that enables highly sensitive, untargeted detection of ctDNA in all cancer patients regardless of tumor hotspot variants. By using distinct cutoffs for detection and quantification of ctDNA in all of our approaches, we can reliably detect residual disease after surgery as well as response or resistance to treatment.
-
-
ELBS Team
Dr. Ariane Hallermayr
Head of Research and Development / Biochemist
Bayerstr. 3-5
80335 Munich
Germany
ariane.hallermayr@mgz-muenchen.de
Dr. Thomas Keßler
NGS data interpretation and reporting / Biologist
Bayerstr. 3-5
80335 Munich
Germany
thomas.kessler@mgz-muenchen.de
Dr. Verena Steinke-Lange
Clinical geneticist
Bayerstr. 3-5
80335 Munich
Germany
Prof. Dr. Elke Holinski-Feder
Management / Clinical geneticist
Bayerstr. 3-5
80335 Munich
Germany
-
Liquid Biopsy Profile
Cancer types Solid tumors (e. g. colorectal cancer, non-small cell lung cancer, breast cancer) Clinical application Treatment decision, detection of minimal residual disease, treatment monitoring, early detection, molecular diagnostics of mosaic diseases Liquid Biopsy Source Blood plasma Technologies available for liquid biopsy Droplet Digital PCR (ddPCR)
Targeted Duplex sequencing
In-house developed pipeline for ctDNA detection based on fragmentation, epigenetic signatures and somatic copy number alterations (LIFE-CNA) including machine learning classifiersBiobank NA Key publications Hallermayr, A., Wohlfrom, T., Steinke-Lange, V.; Benet-Pagès, A.; Scharf, F.; Heitzer, E.; Mansmann, U.; Haberl, C.; de Wit, M.; Vogelsang, H.; Rentsch, M.; Holinski-Feder, E.; Pickl, J.M.A. Somatic copy number alteration and fragmentation analysis in circulating tumor DNA for cancer screening and treatment monitoring in colorectal cancer patients. J Hematol Oncol 2022, 15, 125.
Hallermayr, A.; Steinke-Lange, V.; Vogelsang, H.; Rentsch, M.; de Wit, M.; Haberl, C.; Holinski-Feder, E.; Pickl, J.M.A. Clinical Validity of Circulating Tumor DNA as Prognostic and Predictive Marker for Personalized Colorectal Cancer Patient Management. Cancers 2022, 14, 851.
Hallermayr, A.; Benet-Pagès, A.; Steinke-Lange, V.; Mansmann, U.; Rentsch, M.; Holinski-Feder, E.; Pickl, J.M.A. Liquid Biopsy Hotspot Variant Assays: Analytical Validation for Application in Residual Disease Detection and Treatment Monitoring. Clin. Chem. 2021, 67, 1483–1491.
- NIBSC
- ELBS Team
- Profile Liquid Biopsy Research
-
NIBSC

The National Institute for Biological Standards and Control (NIBSC, UK) is a centre of the MHRA and a global leader in the characterisation, standardisation and control of biological medicines. NIBSC is the world’s major developer and distributor of World Health Organisation (WHO) International Standards (ISs). WHO ISs are the ‘gold standards’ from which countries and manufacturers can calibrate their own working standards for biological testing. WHO ISs are prepared as a single homogeneous batch of several thousand ampoules, are intended to last many years, are not intended for routine use and act as calibrators of assays and secondary standards to enable the harmonization of the measurement of biological activity through traceability to a single common standard. NIBSC has endorsement from the WHO Expert Committee on Biological Standardization to develop WHO ISs for ctDNA. After the initial focus on the development of WHO ISs for EGFR variants (L858R, exon 19 deletions, and T790M) ctDNA and genomic DNA, NIBSC will expand the portfolio to other biomarkers. ctDNA WHO ISs should ideally be commutable with patients’ samples and allow harmonization of variant percentage, DNA fragment size(s), ctDNA yield, and gene copy numbers. Currently NIBSC is assessing the performance of several matrices with various cell-line derived fragmented DNAs to determine the optimal format for the standards, whilst cross-referencing to patient ctDNA materials and other reference materials already on the market.
-
-
ELBS Team
Leandro Lo Cascio
Principal Investigator, Genomics and Genome Engineering
Blanche Lane
South Mimms
Potters Bar
Hertfordshire
EN6 3QG, U.K.
-
Profile Liquid Biopsy Research
Cancer types Initial focus on EGFR variants (L858R, exon 19 deletions, and T790M) in ctDNA and genomic DNA. Clinical application ctDNA and genomic DNA WHO International Standards that mimic clinical specimens Liquid biopsy source Cell line derived fragmented DNA Technologies available for Liquid Biopsy Bulk, Freeze-dried fragmented DNA in plasma, digital PCR, NGS Publications Poster at “Research & Technology Series: Immuno-oncology meeting 10th-11th October 2019, titled: “Development of the First WHO International Standards for ctDNA”
- Netherlands Cancer Institute - COIN-Consortium
- ELBS Team
- Profile Liquid Biopsy Research
-
Netherlands Cancer Institute - COIN-Consortium

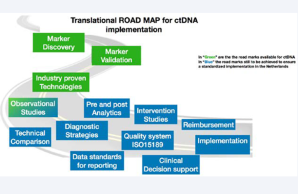
The COIN-consortium is a Dutch initiative in which a nation-wide team of multidisciplinary specialists are working together towards a coordinated clinical implementation of liquid biopsy ctDNA analysis as an innovative form of minimal invasive molecular diagnostics in the healthcare system of The Netherlands.
Over the past years multiple platforms have been developed to detect ctDNA, yielding industry-proven technologies suited for clinical implementation (see roadmap), yet, ctDNA clinical utility remains to be proven. The COIN-project aims to enable coordinated implementation of ctDNA diagnostics in clinical practice. The project consists of two parts. Part 1 aims to establish a generic multidisciplinary framework to guide successful clinical implementation of ctDNA and other future biomarkers. Challenges in (pre-)analysis, reporting, quality assurance, cost-effectiveness, clinical utility, and reimbursement will be addressed making use of the knowledge generated in multiple observational studies. These studies focus on colorectal and non-small cell lung cancer as there is a substantial and active ctDNA research community for these two tumor types. Part 2 of COIN is the first Dutch ctDNA biomarker-driven intervention study, MEDOCC-CrEATE, in which patients with stage II colon cancer will be offered adjuvant chemotherapy based on the presence of ctDNA in their blood after surgery. The intervention arm will be compared to current standard of care. The patient inclusion of MEDOCC-CrEATE started in June 2020.
Contact: COIN@nki.nl
-
-
ELBS Team
Daan van den Broek, PhD
Head Dept of Laboratory Medicine
The Netherlands Cancer Institute /Antoni van Leeuwenhoek
Da.vd.broek@nki.nl
Gerrit Meijer, Prof.
Head Research & Innovation Dept of Pathology; Head Division of Diagnostic Translational Oncology
The Netherlands Cancer Institute /Antoni van Leeuwenhoek
g.meijer@nki.nl
Veerle Coupé, PhD
Chair Decision Modelling Center
Amsterdam UMC
v.coupe@amsterdamumc.nl
Kim Monkhorst, PhD
Pathologist
The Netherlands Cancer Institute /Antoni van Leeuwenhoek
k.monkhorst@nki.nl
Ed Schuuring, Prof.
Head of the Laboratory for Molecular Pathology
UMCG Groningen
e.schuuring@umcg.nl
Marjolijn Ligtenburg, Prof.
Head of the Laboratory Tumor Genetics
Radboud University Medical Center
Marjolijn.Ligtenberg@radboudumc.nl
Ron van Schaik, Prof.
Head Dept. Clinical Chemistry
Erasmus MC University Medical Center Rotterdam
r.vanschaik@erasmusmc.nl
Thijs van Veghel
Clinical Informatician
IKNL, Innovation, Clinical Informatics and Data Science
t.vanvegchel@iknl.nl
Remond Fijneman, PhD
Associate Group Leader
Netherlands Cancer Institute, Pathology
r.fijneman@nki.nl
-
Profile Liquid Biopsy Research
Cancer types Colorectal cancer and non-small cell lung cancer Clinical application Detection of minimal residual disease, treatment response prediction and monitoring, detection of treatment resistant mechanisms Liquid Biobsy Source Blood, plasma Technologies available for liquid biopsy (multiple) PCR-based and NGS-based ctDNA technologies Biobank cell-free plasma (ctDNA), white blood cells (germline DNA), solid tumors (tumor DNA) Key publications Schraa SJ, van Rooijen KL, van der Kruijssen DEW, et al. Circulating tumor DNA guided adjuvant chemotherapy in stage II colon cancer (MEDOCC-CrEATE): study protocol for a trial within a cohort study. BMC Cancer. 2020;20(1):790. Published 2020 Aug 20. doi:10.1186/s12885-020-07252-y
Weber S, Spiegl B, Perakis SO, Ulz CM, Abuja PM, Kashofer K, van der Leest P, Azpurua MA, Tamminga M, Brudzewsky D, Rothwell DG, Mohan S, Sartori A, Lampignano R, Konigshofer Y, Sprenger-Haussels M, Wikman H, Bergheim IR, Kloten V, Schuuring E, Speicher MR, Heitzer E. (2020) Technical evaluation of commercial mutation analysis platforms and reference materials for liquid biopsy profiling. Cancers (Basel) 12, 1588.
Lampignano R, Neumann MHD, Weber S, Kloten V, Herdean A, Voss T, Groelz D, Babayan A, Tibbesma M, Schlumpberger M, Chemi F, Rothwell DG, Wikman H, Galizzi JP, Bergheim IR, Russnes H, Mussolin B, Bonin S, Voigt C, Musa H, Pinzani P, Lianidou E, Brady G, Speicher MR, Pantel K, Betsou F, Schuuring E, Kubista M, Ammerlaan W, Sprenger-Haussels M, Schlange T, Heitzer E. (2020) Multicenter evaluation of circulating cell-free DNA extraction and downstream analyese for the development of standardized (pre)analytical work flows. Clin Chem 1, 149–160.
van 't Erve I, Greuter MJE, Bolhuis K, Vessies DCL, Leal A, Vink GR, van den Broek D, Velculescu VE, Punt CJA, Meijer GA, Coupé VMH, Fijneman RJA. Diagnostic Strategies toward Clinical Implementation of Liquid Biopsy RAS/BRAF Circulating Tumor DNA Analyses in Patients with Metastatic Colorectal Cancer. J Mol Diagn. 2020 Sep 19:S1525-1578(20)30462-1. doi: 10.1016/j.jmoldx.2020.09.002. Online ahead of print. PMID: 32961317
van den Broek D, Hiltermann TJN, Biesma B, Dinjens WNM, 't Hart NA, Hinrichs JWJ, Leers MPG, Monkhorst K, van Oosterhout M, Scharnhorst V, Schuuring E, Speel EM, van den Heuvel MM, van Schaik RHN, von der Thüsen J, Willems SM, de Visser L, Ligtenberg MJL. Implementation of Novel Molecular Biomarkers for Non-small Cell Lung Cancer in the Netherlands: How to Deal with Increasing Complexity. Front Oncol. 2020 Jan 22;9:1521. doi: 10.3389/fonc.2019.01521. eCollection 2019. PMID: 32039011
- Pasteur Hospital
- ELBS Team
- Profile Liquid Biopsy Research
-
Pasteur Hospital


The Laboratory of Clinical and Experimental Pathology (LPCE) (Pasteur Hospital, University Côte d’Azur) founded in 2006 has an expertise mainly in lung and thyroid cancers and in melanoma. This laboratory developed pathology and biology diagnosis using different approaches (pathological and cytological diagnosis, immunohistochemistry, in situ hybridization and molecular biology using targeted or next generation sequencing). This laboratory is associated with the FHU OncoAge (www.oncoage.org) and the Institute for Ageing and Cancer of Nice (IRCAN, www.ircan.org). Moreover, a dedicated biobank has been set up in the LPCE (www.biobank-cotedazur) mainly focus on blood and tissue samples provided by patients with lung cancer, melanoma and thyroid diseases. A specific training program in biobanking has been created in 2016 (MSc Biobanks and Complex data Management, http://web.univ-cotedazur.fr//en/education/informations-utiles/les-informations-utiles/biobanks-complex-data). A liquid biopsy unit has been developed at the LPCE in 2009 in order to organize translational research programs which have been granted by the French NCI. Moreover, the liquid biopsy unit takes responsability for the detection of different predictive biomarkers in daily practice (EGFR mutations, ALK rearangement, BRAF/NRAS mutations, etc.) and participates to different clinical trials using both specific based PCR sequencing and next generation sequencing approaches with gene panels of different sizes.
-
-
ELBS Team

Prof. Paul Hofman, MD, PhD
Head of the Laboratory of Clinical and Experimental Pathology
BP 69, Nice
France
Hofman.p@chu-nice.fr

Prof Marius Ilié, MD, PhD
Deputy Director, Laboratory of Clinical and Experimental Pathology
BP 69, Nice
France
Ilie.m@chu-nice.fr

Dr Véronique Hofman, MD, PhD
Head of the CTC programs at the LPCE
BP 69, Nice
France
Hofman.v@chu-nice.fr
-
-
Profile Liquid Biopsy Research
Cancer types Lung cancer, melanoma (skin and ocular melanoma) & thyroid cancer Clinical application Early detection of lung cancer
Detection of predictive biomarkers
Identification of resistance mechanismsLiquid Biobsy Source Whole blood, plasma, sera, PBMCs, urines, saliva Technologies available for liquid biopsy CTC: CellSearch, ISET, Vortex,
cfDNA: NGS S5, Genereader, COBAS, IDYLLABiobank www.biobank-cotedazur.fr : Lung and thyroid cancers and melanoma (tissue and liquid samples) Key publications Marquette CH, Boutros J, Benzaquen J, Ferreira M, Pastre J, Pison C, Padovani B, Bettayeb F, Fallet V, Guibert N, Basille D, Ilie M, Hofman V, Hofman P; AIR project Study Group. Circulating tumour cells as a potential biomarker for lung cancer screening: a prospective cohort study. Lancet Respir Med. 2020 Jul;8(7):709-716.
Heeke S, Hofman V, Ilié M, Allegra M, Lespinet V, Bordone O, Benzaquen J, Boutros J, Poudenx M, Lalvée S, Tanga V, Salacroup C, Bonnetaud C, Marquette CH, Hofman P. Prospective evaluation of NGS-based liquid biopsy in untreated late stage non-squamous lung carcinoma in a single institution. J Transl Med. 2020 Feb 17;18(1):87.
Hofman P, Heeke S, Alix-Panabières C, Pantel K. Liquid biopsy in the era of immuno-oncology: is it ready for prime-time use for cancer patients? Ann Oncol. 2019 Sep 1;30(9):1448-1459.
Calabrese F, Lunardi F, Pezzuto F, Fortarezza F, Vuljan SE, Marquette C, Hofman P. Are There New Biomarkers in Tissue and Liquid Biopsies for the Early Detection of Non-Small Cell Lung Cancer? J Clin Med. 2019 Mar 26;8(3):414.
Ilié M, Szafer-Glusman E, Hofman V, Chamorey E, Lalvée S, Selva E, Leroy S, Marquette CH, Kowanetz M, Hedge P, Punnoose E, Hofman P. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol. 2018 Jan 1;29(1):193-199.
- Queen Mary University of London
- ELBS Team
- Profile Liquid Biopsy Research
-
Queen Mary University of London

Queen Mary University of London is a leading research-intensive University, where we believe that when views collide, disciplines interact, and perspectives intersect, truly original thought takes form. Queen Mary is one of 24 leading UK universities represented by the Russell Group, that are committed to maintaining the very best research, an outstanding teaching and learning experience, excellent graduate employability and unrivalled links with businesses and the public sector. The results of the most recent national assessment of research – the Research Excellence Framework 2021 – have confirmed our place in the very top group of research-led universities in the UK. We were ranked 7th in the UK among multi-faculty universities, with 92% of our research assessed as internationally excellent or world-leading.
Barts Cancer Institute (BCI) is part of the Faculty of Medicine and Dentistry at Queen Mary University of London, and is one of the top five cancer research centres in the UK. Our overriding objective is to ensure that our research, which aims to identify ways to prevent cancer and develop better diagnostics and treatments, is relevant to and will have a significant impact on all cancer patients. Together with the Centre for Cancer Prevention at Queen Mary’s Wolfson Institute of Population Health, BCI forms the Cancer Research UK (CRUK) Barts Centre – one of 15 Cancer Research UK Centres of Excellence.
-
-
ELBS Team

Prof. Yong-Jie Lu MD, PhD
Professor in Molecular Oncology
Queen Mary University of London
John Vane Science Centre
Charterhouse Square, LONDON EC1M 6BQ
Barts Cancer Institute – a Cancer Research UK Centre of Excellence
Centre for Cancer Biomarkers and Biotherapeutics
Honorary Principle Investigator, Barts Health NHS Trust
Prof. Claude Chalala
Professor of Bioinformatics
Queen Mary University of London
Joseph Rotblat Building, Charterhouse Square, London EC1M 6BQ
Barts Cancer Institute – a Cancer Research UK Centre of Excellence
Centre for Cancer Biomarkers and Biotherapeutics
Deputy Centre Lead
Honorary Researcher, Barts Health NHS Trust
Tel. +44 (0)20 7882 3570
c.chelala@qmul.ac.uk
About Prof. Claude Chalala
Prof. Tatjana Crnogorac-Jurcevic, MD, PhD
Professor of Molecular Pathology and Biomarkers
Queen Mary University of London
Joseph Rotblat Building, Charterhouse Square, London EC1M 6BQ, UK
Barts Cancer Institute – a Cancer Research UK Centre of Excellence
Centre for Cancer Biomarkers and BiotherapeuticsTel. +44 (0)20 7882 3554
t.c.jurcevic@qmul.ac.uk
About Prof. Tatjana Crnogorac-Jurcevic -
-
Profile Liquid Biopsy Research
Cancer types Male urological (prostate, testicular, bladder, renal penile), breast, ovarian, cervical and digestive system (pancreatic, colorectal, gastric, oesophageal) cancers and haematological malignancies. Clinical application Diagnostic pathway improvement, early cancer detection, prognosis, treatment response prediction and monitoring. Liquid Biopsy Source Blood, PBMC, flow-sorted immune cells, plasma (for ctDNA, EX/exosome, proteins), platelets and/or urine. Technologies available for liquid biopsy Parsortix for CTC isolation, Illumine NGS, 10X single RNA-seq, Fluidigm multiplex qPCR. Biobank Urological, GI system, breast and ovarian cancers. Key publications Lawrence R, Watters E, Davies CR, Pantel K, Lu YJ. Circulating tumour cells for early detection of clinically relevant cancer. Nat Rev Clin Oncol. In press. 2023.
Xu L, Mao X, Grey A, Scandura G, Guo T, Burke E, Marzec J, Abdu S, Stankiewicz E, Davies CR, Rajan P, Tipples K, Hines J, Chan PY, Campbell D, Wilkinson K, Kudahetti S, Shamash J, Oliver T, Berney D, Shaw G, Lu YJ. Non-invasive Detection of Clinically Significant Prostate Cancer Using Circulating Tumor Cells. J Urol. 203(1):73-82; 2020.
Xu L, Mao X, Guo T, Chan PY, Shaw G, Hines J, Stankiewicz E, Wang Y, Oliver RTD, Ahmad AS, Berney D, Shamash J, Lu YJ. The novel association of circulating tumor cells and circulating megakaryocytes with prostate cancer prognosis. Clin Cancer Res. 23(17):5112-22; 2017.
Debernardi S, Blyuss O, Rycyk D, Srivastava K, Jeon CY, Cai H, Cai Q, Shu XO, Crnogorac-Jurcevic T. Urine biomarkers enable pancreatic cancer detection up to 2 years before diagnosis. Int. J. Cancer. 152:769–780; 2023.
Sivapalan, L., G. J. Thorn, E. Gadaleta, H. M. Kocher, H. Ross-Adams, and C. Chelala. 'Longitudinal profiling of circulating tumour DNA for tracking tumour dynamics in pancreatic cancer', BMC Cancer, 22: 369. 9. 2022.
- Stavanger University Hospital
- ELBS Team
- Profile Liquid Biopsy Research
-
Stavanger University Hospital


Stavanger University Hospital is the only hospital in the large area of south Rogaland on the west coast of Norway. The hospital covers a population of approximately 460.000 inhabitants; the hospital has 785 beds and nearly 8000 employees. Research is one of the main four tasks of the hospital, and cancer is one of the major diagnoses treated.
The main research areas at the Department of Hematology and Oncology are translational research, clinical trials and development of new treatment strategies. The main focus for the micrometastasis research group at the department is translational research on breast and pancreatic cancer, with a special focus on circulating/disseminated tumor cells and circulating tumor DNA detection. The micrometastasis group has a broad collaboration including both national and international partners, like Karolinska Institutet and the University of Texas M.D. Anderson Cancer Center and was recently acknowledged to be part of The Norwegian Cancer Society’s national expert group on pancreatic cancer. Our micrometastasis research group is located in the Laboratory for molecular biology, a modern multi-disciplinary core facility for cancer research, founded in 2007, with state-of-the art infrastructure and instruments that facilitates a wide range of molecular analyses.
-
-
ELBS Team

Bjørnar Gilje (MD, PhD)
Head at Department of Hematology and Oncology
Gerd-Ragna Bloch Thorsens gate 8,
4019 Stavanger,
Norway
bjornar.gilje@sus.no

Oddmund Nordgård (PhD)
Chief engineer/Professor
Gerd-Ragna Bloch Thorsens gate 8,
4019 Stavanger,
Norway
oddmund.nordgard@sus.no

Kjersti Tjensvoll (PhD)
Chief engineer/Researcher
Gerd-Ragna Bloch Thorsens gate 8,
4019 Stavanger,
Norway
kjersti.tjensvoll@sus.no
-
-
Profile Liquid Biopsy Research
Cancer types Breast and pancreatic cancer Clinical application Early detection of relapse, disease monitoring, monitoring of therapy response Liquid Biobsy Source Blood, plasma, bone marrow Technologies available for liquid biopsy Next generation sequencing (Ion Chief, S5, Proton), digital droplet PCR, LightCycler 480, Flow cytometry, Immunofluoresence microscopy with cell picking. Biobank Liquid biopsies (DNA/RNA from CTCs, plasma, bone marrow)
The PACT-ACT biobank contains blood and plasma samples collected from advanced pancreatic cancer patients before initiation of treatment and every months during chemotherapy, as well as bone marrow samples collected before and after treatment start (PI: Bjørnar Gilje).
The Prospective Breast Cancer Biobank (PBCB), a regional biobank containing blood, urine and plasma/serum samples collected every 6/12 months for 11 years from 1100 patients with early breast cancer. Patients reported outcome measure (PROM) are also collected, yearly (PIs: Gunnar Mellgren and Håvard Søiland)Key publications Tjensvoll K, Nordgård O, Skjæveland M, Oltedal S, Janssen EAM, Gilje B. Detection of disseminated tumor cells in bone marrow predict late recurrences in operable breast cancer patients. BMC Cancer. 2019 Nov 21;19(1):1131
Lapin M, Oltedal S, Tjensvoll K, Buhl T, Smaaland R, Garresori H, Javle M, Glenjen NI, Abelseth BK, Gilje B, Nordgård O. Fragment size and level of cell-free DNA provide prognostic information in patients with advanced pancreatic cancer. Transl Med. 2018 Nov 6;16(1):300
Nordgård O, Tjensvoll K, Gilje B, Søreide K. Circulating tumour cells and DNA as liquid biopsies in gastrointestinal cancer. Br J Surg. 2018 Jan;105(2):e110-e120. Review
Lapin M, Tjensvoll K, Oltedal S, Buhl T, Gilje B, Smaaland R, Nordgård O. MINDEC-An Enhanced Negative Depletion Strategy for Circulating Tumour Cell Enrichment. Sci Rep. 2016 Jul 19;6:28929
Tjensvoll K, Lapin M, Buhl T, Oltedal S, Steen-Ottosen Berry K, Gilje B, Søreide JA, Javle M, Nordgård O, Smaaland R. Clinical relevance of circulating KRAS mutated DNA in plasma from patients with advanced pancreatic cancer. Mol Oncol. 2016 Apr;10(4):635-43
- Toulouse University Cancer Institute-Oncopole
- ELBS Team
- Profile Liquid Biopsy Research
-
Toulouse University Cancer Institute-Oncopole



The Oncopole site of Toulouse University Cancer Institute (IUCT-O), together with Cancer Research Center of Toulouse (CRCT), is a Comprehensive Cancer Center member of Organization of European Cancer Institutes (OECI) offering pioneering therapies and technologies. IUCT‑O has three missions, treatment, research (clinical trials, care management and translational research) and teaching, IUCT-located in the heart of a campus grouping together public and private stakeholders involved in the fight against cancer from basic science to clinical trials. The Prospective Biology Unit within the Medical Laboratory of IUCT-O is dedicated to clinical research, innovation and medico-economic evaluation of liquid biopsies in cancers. Together with clinicians and CRCT research teams and the support of CRCT technology cluster (cell imaging, genomic and transcriptomic with single cell analysis …), we take an active part in translational research and clinical trials. Our projects focus on the development of new circulating biological markers for diagnosis, prognosis, longitudinal follow-up of the disease and prediction of therapeutic response, with the molecular analysis of circulating tumor DNA, enrichment and phenotypic characterization of circulating tumor cells, quantification of circulating miRNAs, using innovative technologies such as digital droplet PCR for ctDNA analysis, ISET technology for CTC enrichment or nCounter Analysis System (Nanostring) for miRNA assays.
-
-
ELBS Team

Prof. Gilles Favre
Director of the Cancer Research Center of Toulouse
1 av Irene Joliot-Curie
31059 Toulouse cedex 9
France
favre.gilles@iuct-oncopole.fr

Anne Pradines, PhD
Prospective Biology Unit leader
1 av Irene Joliot-Curie
31059 Toulouse cedex 9
France
pradines.anne@iuct-oncopole.fr
-
-
Profile Liquid Biopsy Research
Cancer types Solid tumors (lung, melanoma, breast, ovarian cancer, prostate) Clinical application Early detection of primary cancer and relapse, monitoring of therapy response, identification of therapy targets and resistance mechanisms Liquid Biobsy Source Blood, plasma Technologies available for liquid biopsy ddPCR, Target re-sequencing Biobank Plasma, PBMC Key publications Guibert N, Pradines A, Favre G, Mazieres J. Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages. Eur Respir Rev. 2020;29(155):190052.
Guibert N, Jones G, Beeler JF, et al. Targeted sequencing of plasma cell-free DNA to predict response to PD1 inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2019;137:1–6
Keller L, Guibet N, Delaunay M, Casanova A, Farella M, Brayer S, Gilhodes J, Martin E, Favre G, Pradines A, Meyer N. Early ctDNA variation predicts tumor response in melanoma patients treated with immunotherapy. Acta Dermato 2019 ; 99: 206–210.
Guibert N, Delaunay M, Lusque A, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer. 2018;120:108–112.
Guibert N, Pradines A, Farella M, Casanova A, Gouin S, Keller L, Favre G, Mazieres J. Monitoring KRAS mutations in circulating DNA and tumor cells using digital droplet PCR during treatment of KRAS-mutated lung adenocarcinoma. Lung Cancer 2016; 100: 1-4.
- University Cancer Center Schleswig-Holstein
- ELBS Team
- Profile Liquid Biopsy Research
-
University Cancer Center Schleswig-Holstein

„Molecular Therapies“ group at the University Cancer Center Schleswig-Holstein, Campus Lübeck
Our research focus includes preclinical and clinical development of targeted treatment strategies in cancer, molecular mechanisms of therapy resistance and disease persistence, and nucleic acid-based biomarkers. In the past, we elucidated molecular mechanisms leading to resistance to targeted cancer treatment in leukemia and solid tumors, and developed strategies to prevent and overcome treatment resistance. More recently, we conduct clinical trials establishing PCR-based liquid biopsy techniques for early treatment monitoring and prediction of relapse in melanoma, gastrointestinal stromal tumor and lung cancer. Using informative disease models, we identified novel treatment targets in leukemia and solid tumors, including CSF2RB as in FLT3-ITD positive acute myeloid leukemia. We put a strong focus on implementing a standardized molecular, genetic and bioinformatic workflow for precision oncology in a clinical setting and contributed in establishing a Molecular Tumor Board (MTB) at the University Cancer Center Schleswig-Holstein.
-
-
ELBS Team
Prof. Dr. Nikolas von Bubnoff
Head of the Molecular Therapies WG
Nikolas.vonbubnoff@uksh.de
-
Profile Liquid Biopsy Research
Cancer types melanoma, GIST, colon-, pancreas- carcinoma, lung, bladder, mastocytosis, choroidal melanoma Clinical application therapy monitoring, diagnostics, prediction of relapse Liquid Biopsy Source Blood, bile, urine, bone marrow aspirat (ctDNA, gDNA, EVs) Technologies available for liquid biopsy FACS, Nanoparticle Tracking Analysis, Digital Droplet PCR Biobank Broad Consent, Sample collection and transport (surgery, clinical departments, pathology), sample processing of fluid and solid samlpes (automated aliquoting of serum, plasma, urine; PBMC isolation; automated DNA extraction, tissue preparation), sample storage (-80°C, automated cryostorage (-190°C)), data management (CentraXX), certified QM System (DIN EN ISO 9001:2015) Key publications Charlet A, Kappenstein M, Keye P, Kläsener K, Endres C, Poggio T, Gorantla SP, Kreutmair S, Sänger J, Illert AL, Miething C, Reth M, Duyster J, Rummelt C, von Bubnoff N. The IL-3, IL-5, and GM-CSF common receptor beta chain mediates oncogenic activity of FLT3-ITD-positive AML. Leukemia. 2021 Nov 8. doi: 10.1038/s41375-021-01462-4. PMID: 34750506.
Waldeck S, Mitschke J, Wiesemann S, Rassner M, Andrieux G, Deuter M, Mutter J, Lüchtenborg AM, Kottmann D, Titze L, Zeisel C, Jolic M, Philipp U, Lassmann S, Bronsert P, Greil C, Rawluk J, Becker H, Isbell L, Müller A, Doostkam S, Passlick B, Börries M, Duyster J, Wehrle J, Scherer F, von Bubnoff N. Early assessment of circulating tumor DNA after curative-intent resection predicts tumor recurrence in early-stage and locally advanced non-small-cell lung cancer. Mol Oncol. 2021 Oct 15. doi: 10.1002/1878-0261.13116. PMID: 34653314.
Hoefflin R, Lazarou A, Hess ME, Reiser M, Wehrle J, Metzger P, Frey AV, Becker H, Aumann K, Berner K, Boeker M, Buettner N, Dierks C, Duque-Afonso J, Eisenblaetter M, Erbes T, Fritsch R, Ge IX, Geißler AL, Grabbert M, Heeg S, Heiland DH, Hettmer S, Kayser G, Keller A, Kleiber A, Kutilina A, Mehmed L, Meiss F, Poxleitner P, Rawluk J, Ruf J, Schäfer H, Scherer F, Shoumariyeh K, Tzschach A, Peters C, Brummer T, Werner M, Duyster J, Lassmann S, Miething C, Boerries M, Illert AL, von Bubnoff N. Transitioning the Molecular Tumor Board from Proof of Concept to Clinical Routine: A German Single-Center Analysis. Cancers (Basel). 2021 Mar 8;13(5):1151. doi: 10.3390/cancers13051151. PMID: 33800365; PMCID: PMC7962829.
Zeiser R*, von Bubnoff N*, Butler J, Mohty M, Niederwieser D, Or R, Szer J, Wagner EM, Zuckerman T, Mahuzier B, Xu J, Wilke C, Gandhi KK, Socié G; REACH2 Trial Group. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N Engl J Med. 2020 May 7;382(19):1800-1810. * equal contribution.
Braune J, Keller L, Schiller F, Graf E, Rafei-Shamsabadi D, Wehrle J, Follo M, Philipp U, Hussung S, Pfeiger D, Mix M, Duyster J, Fritsch R, von Bubnoff D, Meiss F, von Bubnoff N. Circulating Tumor DNA Allows Early Treatment Monitoring in BRAF- and NRAS-Mutant Malignant Melanoma. JCO Precision Oncology 2020, 4 (2020) 20-31.
- University Hospital Essen
- ELBS Team
- Profile Liquid Biopsy Research
-
University Hospital Essen

The laboratories at the Department of Dermatology at the University Hospital Essen are primarily focused on the diagnosis and therapy of skin tumors with the goal of transferring research results directly into the clinic. The main research focus is on malignant melanoma, a malignant tumor that originates from pigment producing cells of the skin called melanocytes. Melanoma is an aggressive tumor with dramatically reduced patient survival when disseminated to distant organs. Despite the historic success of modern cancer drugs, the cure of patients is hampered by the dramatic tumor heterogeneity and plasticity of melanoma leading to therapy failure in most cases and limited diagnostic options to monitor therapy response/resistance over time. The Tumor Heterogeneity and Plasticity lab (headed by Prof. Dr. med. Alexander Roesch) has dedicated reasearch projects towards finding ways to overcome the intrinsic therapy resistance of melanoma cell subpopulations in vitro and in vivo. Specifically, the lab focuses on the characterization of tumor heterogeneity, phenotypic plasticity of melanoma cell subpopulations, bioenergetic metabolism, and histone 3 K4 demethylases. Translational research questions are concentrated on how to overcome inter- and intra-tumor heterogeneity in patients. Liquid biopsies, comprising of the noninvasive analysis of circulating tumor-derived material in the blood, represent an innovative tool in precision oncology and provide a potential way to overcome current limitations associated with tissue biopsies.
For further details feel free to visit our department’s website and follow our group on Twitter (@LabRoesch).
-
-
ELBS Team

Prof. Dr. med. Alexander Roesch
Group leader of Tumor Heterogeneity and Plasticity
Hufelandstrasse 55
45147 Essen, Germany
alexander.roesch@uk-essen.de

Dr.rer.nat. Renata Varaljai
Postdoctoral scientist
Hufelandstrasse 55
45147 Essen, Germany
renata.varaljai@uk-essen.de
-
-
Profile Liquid Biopsy Research
Cancer types Melanoma Clinical application Monitoring of therapy response, early detection of relapse and resistance Liquid Biobsy Source Blood, plasma Technologies available for liquid biopsy qPCR, digital PCR Biobank SCABIO (Skin Cancer Biobank) Key publications Liquid Biopsy
Renáta Váraljai, Salma Elouali, Smiths S. Lueong, Kilian Wistuba-Hamprecht, Teofila Seremet, Jens T. Siveke, Jürgen. C. Becker, Antje Sucker, Annette Paschen, Peter A. Horn, Bart Neyns, Benjamin Weide, Dirk Schadendorf, Alexander Roesch: The predictive and prognostic significance of cell-free DNA concentration in melanoma. JEADV, DOI: 10.1111/jdv.16766 (2020)
Martin Metzenmacher, Renáta Váraljai, Balazs Hegedüs, Igor Cima, Jan Forster, Alexander Schramm, Björn Scheffler, Peter Horn, Christoph Klein, Tibor Szarvas, Henning Reis, Nicola Bielefeld, Alexander Roesch, Clemens Aigner, Volker Kunzmann, Marcel Wiesweg, Jens Siveke, Martin Schuler, Smiths Lueong: Plasma next generation sequencing and droplet digital-qPCR-based quantification of circulating cell-free RNA for non-invasive early detection of cancer. Cancers, 02/2020; 12(2), 353, DOI: 10.3390/cancers12020353 (2020)
Renáta Váraljai, Kilian Wistuba-Hamprecht, Teofila Seremet, Joey Mark S. Diaz, Jérémie Nsengimana, Antje Sucker, Jan-Malte Placke, Peter A. Horn, Nils von Neuhoff, Batool Shannan, Heike Chauvistré, Felix C. E. Vogel, Susanne Horn, Jürgen C. Becker, Julia Newton-Bishop, Andreas Stang, Bart Neyns, Benjamin Weide, Dirk Schadendorf, Alexander Roesch: Application of circulating cell-free tumor DNA profiles for therapeutic monitoring and outcome prediction in genetically heterogeneous metastatic melanoma. JCO Precision Oncology, DOI: 10.1200/PO.18.00229 (2019)
Other translational projects
Jan C. Brase, Robert F. H. Walter, Alexander Savchenko, Daniel Gusenleitner, James Garrett, Tobias Torsten Schimming, Renáta Váraljai, Deborah Castelletti, Ju-Young Kim, Naveen K Dakappagari, Ken Schultz, Caroline Robert, Georgina V. Long, Dr. Paul D Nathan, Antoni Ribas, Keith T. Flaherty, Boguslawa Karaszewska, Jacob Schachter, Antje Sucker, Kurt W Schmid, Lisa Zimmer, Elisabeth Livingstone, Eduard Gasal, Dirk Schadendorf, Alexander Roesch: Role of Tumor-Infiltrating B Cells in Clinical Outcome of Patients with Melanoma Treated With Dabrafenib Plus Trametinib. Clin Cancer Res, June 9 2021 DOI: 10.1158/1078-0432.CCR-20-3586
B. Shannan, J. Matschke, H. Chauvistré, F. Vogel, D. Klein, F. Meier, D. Westphal, J. Bruns, R. Rauschenberg, J. Utikal, A. Forschner, C. Berking, P. Terheyden, E. Dabrowski, R. Gutzmer, D. Rafei-Shamsabadi, F. Meiss, L. Heinzerling l, L. Zimmer, Elisabeth Livingstone, R. Váraljai, A. Hoewner, S. Horn, J. Klode, M. Stuschke, B. Scheffler, A. Marchetto, G. Sannino,T.G.P. Gruenewald, D. Schadendorf, V. Jendrossek, A. Roesch: Sequence-dependent cross-resistance of combined radiotherapy plus BRAFV600E inhibition in melanoma. Eur J Cancer, 03/2019; Volume 109, Pages 137-153
- University Medical Center Hamburg-Eppendorf
- ELBS Team
- Profile Liquid Biopsy Research
-
University Medical Center Hamburg-Eppendorf
 The Liquid Biopsy Translational Research Network at UKE
The Liquid Biopsy Translational Research Network at UKE
It is part of the Univerity Cancer Center Hamburg (UCCH), one of the top cancer center ("Onkologisches Spitzenzentren") Funding
Funding
Individual projects are funded by highly competitive EU grants (ERC, IMI, ERA-Net Transcan, Marie Curie-Sklodowaska Training network).The Liquid Biopsy Translational Research Network at UKE has been established by Klaus Pantel and is part of the University Cancer Center Hamburg (UCCH, Director: Prof. Carsten Bokemeyer), one of the top cancer centers („Onkologische Spitzenzentren“) funded by the German Cancer Aid („Deutsche Krebshilfe“) which also funds a novel post-graduate training center for clinical and translational scientists („Mildred-Scheel Nachwuchszentrum“) at UCCH focusing on cancer cell dissemination/metastasis and liquid biopsy. The network has published more than 300 reports (mostly on clinical studies in patients with various types of solid tumors) and individual projects are funded by highly competitive EU grants (ERC, IMI, ERA-Net TRANSCAN, Marie Curie-Sklodowska Training networks).
-
-
ELBS Team

Prof. Klaus Pantel
Chairman of Institut of Tumorbiologie
Martinistr. 52
20246 Hamburg
Germany -
-
Profile Liquid Biopsy Research
Cancer types Solid tumors (e.g., prostate, breast, lung, GI-tract, melanoma, bladder, cervical cancer) Clinical application Early detection of primary cancer and relaps (MRD), monitoring of therapy response, identification of therapy targets and resistance mechanisms Liquid Biopsy source Blood, plasma, bone marrow Technologies available for Liquid Biopsy CellSearch; Parsortix; others Biobank Solid tumors (CTCs, plasma) Key publications Keller L, Pantel K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat Rev Cancer 2019;19: 553-67.
Pantel K, Alix-Panabieres C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16: 409-24.
Bidard FC, Michiels S, Riethdorf S, Mueller V, Esserman LJ,...Pierga JY, Pantel K. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysis. JNCI. 2018.
Werner S, Brors B, Eick J, Marques E, Pogenberg V, Parret A, Kemming D, Wood AW, Edgren H, Neubauer H, Streichert T, Riethdorf S, Bedi U, Baccelli I, Jucker M, Eils R, Fehm T, Trumpp A, Johnsen SA, Klefstrom J, Wilmanns M, Muller V, Pantel K*, Wikman H*. Suppression of early hematogenous dissemination of human breast cancer cells to bone marrow by retinoic Acid-induced 2. Cancer Discov. 2015;5: 506-19. (*shared senior authorship)
Muller C, Holtschmidt J, Auer M, Heitzer E, Lamszus K, Schulte A, Matschke J, Langer-Freitag S, Gasch C, Stoupiec M, Mauermann O, Peine S, Glatzel M, Speicher MR, Geigl JB, Westphal M, Pantel K*, Riethdorf S. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med. 2014;6: 247ra101. (*shared senior authorship)
- University General Hospital of Heraklion (UoC)
- ELBS Team
- Profile Liquid Biopsy Research
-
University General Hospital of Heraklion (UoC)
/heraklion-logo_contentbild2.png)
/heraklion-building_contentbild2.png)
The University of Crete (UoC) is participating in ELBS with the Laboratory of Translational Oncology (LTO), School of Medicine, UoC and the affiliated Department of Medical Oncology (DMO), University General Hospital of Heraklion, Crete. The DMO is the reference center for patients with cancer for the area of Crete, Greece since it receives approximately 1.000 new cancer patients per year and follows almost 10.000 cancer patients. The mission of the DMO is to provide clinical care to patients with cancer, to perform basic and clinical research, and to provide education and training to the next generation of scientists working on the biology and treatment of cancer. The DMO has expertise in treating patients with different cancer types, particularly focusing on breast, lung, head-neck, gastrointestinal cancers, and melanoma.
The LTO is a bridge by which basic scientists, clinical and translational investigators, and clinical oncologists interact, collaborate, and pool their collective expertise to make significant advances in cancer care and research. LTO has all the equipment needed for performing research activities that make use of liquid biopsy-based biomarkers, including enrichment and phenotypic characterization of circulating tumor cells (CTCs), molecular profiling of circulating tumor DNA, gene expression analysis using peripheral immune cells (PBMCs), and circulating miRNAs.
-
-
ELBS Team
Dr. Dimitrios Mavroudis
MD, PhD, Prof. of Medical Oncology
Head of the Department of Medical Oncology and Head of the Laboratory of Translational OncologyDr. Sofia Agelaki
MD, PhD, Prof. of Medical Oncology
Dr. John Sougklakos
MD, PhD, Prof. of Medical Oncology
The medical staff of the Department of Medical Oncology
consists of 10 highly trained Medical Oncologists and 8 fellows in Medical Oncology.The investigators at Laboratory of Translational Oncology
consist of a team of 4 experienced post-doctoral researchers, 2 PhD students and 2 technicians.
Post-Doctoral Researchers:
Maria A. Papadaki
Kleita Michaelidou
Maria Sfakianaki
Eleni Politaki
-
Profile Liquid Biopsy Research
Cancer types Solid tumors (e.g. Breast cancer, Lung cancer, Colorectal cancer, Melanoma, Pancreatic cancer, Uveal melanoma, Head and neck cancer) Clinical application Prognosis / Prediction of treatment response/ Disease monitoring/ Early detection of relapse/Resistance mechanisms Liquid Biopsy Source Plasma, PBMCs, cfDNA/CTCs Technologies available for liquid biopsy Droplet Digital PCR (QX200 System BioRad), Thermo ABI ViiA 7 Real-Time PCR System, Parsortix™ Cell Separation System, Idylla Biocartis platform Biobank Currently no Biobank. Key publications Papadaki MA, Monastirioti A, Apostolopoulou CA, Aggouraki D, Papadaki C, Michaelidou K, Vassilakopoulou M, Alexakou K, Mavroudis D, Agelaki S. TLR4 and pSTAT3 Expression on Circulating Tumor Cells (CTCs) and Immune Cells in the Peripheral Blood of Breast Cancer Patients: Prognostic Implications. Cancers (Basel). 2022 Feb 18;14(4):1053. doi: 10.3390/cancers14041053. PMID: 35205801
Monastirioti A, Papadaki C, Kalapanida D, Rounis K, Michaelidou K, Papadaki MA, Mavroudis D, Agelaki S. Plasma-Based microRNA Expression Analysis in Advanced Stage NSCLC Patients Treated with Nivolumab. Cancers (Basel). 2022 Sep 28;14(19):4739. doi: 10.3390/cancers14194739. PMID: 36230658
Messaritakis I, Koulouridi A, Boukla E, Sfakianaki M, Vogiatzoglou K, Karagianni M, Gouvas N, Tsiaoussis J, Xynos E, Athanasakis E, Mavroudis D, Tzardi M, Souglakos J. Investigation of Microbial Translocation, TLR and VDR Gene Polymorphisms, and Recurrence Risk in Stage III Colorectal Cancer Patients. Cancers (Basel). 2022 Sep 10;14(18):4407. doi: 10.3390/cancers14184407.
Papadaki MA, Sotiriou AI, Vasilopoulou C, Filika M, Aggouraki D, Tsoulfas PG, Apostolopoulou CA, Rounis K, Mavroudis D, Agelaki S. Optimization of the Enrichment of Circulating Tumor Cells for Downstream Phenotypic Analysis in Patients with Non-Small Cell Lung Cancer Treated with Anti-PD-1 Immunotherapy. Cancers (Basel). 2020 Jun 12;12(6):1556. doi: 10.3390/cancers12061556.
Michaelidou K, Koutoulaki C, Mavridis K, Vorrias E, Papadaki MA, Koutsopoulos AV, Mavroudis D, Agelaki S. Detection of KRAS G12/G13 Mutations in Cell Free-DNA by Droplet Digital PCR, Offers Prognostic Information for Patients with Advanced Non-Small Cell Lung Cancer. Cells. 2020 Nov 20;9(11):2514. doi: 10.3390/cells9112514. PMID: 33233668
- University of Gothenburg
- ELBS Team
- Profile Liquid Biopsy Research
-
University of Gothenburg

University of Gothenburg is among the largest universities in Northern Europe (approx. 47,000 students and 6,000 employees). With eight faculties, University of Gothenburg is also the most wide-ranging and versatile university in Sweden. University of Gothenburg is an active international university engaged in collaborative projects and partnerships globally. Institute of Biomedicine at Sahlgrenska Academy, the medical faculty at University of Gothenburg, has an extensive involvement in education and research. The research activities are many and diverse, but primarily fall into one of four key areas: Infection and Immunology, Cancer and Stem Cell Biology, Genetics and Molecular Medicine, and Cell and Molecular Biology including Glycobiology. The institute actively strives to create environments for translational research, promoting constellations of pre-clinical and clinical groups with extensive international collaborations.
-
-
ELBS Team

Anders Ståhlberg, Associate Professor
Group Leader and Head of Translational Genomics Platform
Sahlgrenska Center for Cancer Research
Medicinaregatan 1F
413 90 Gothenburg
Sweden
anders.stahlberg@gu.se
-
-
Profile Liquid Biopsy Research
Cancer types Solid tumors Clinical application Monitoring of treatment efficacy and early detection of therapy resistance and relapse Liquid Biobsy Source Blood, plasma, urine Technologies available for liquid biopsy Methods for cell-free DNA-, RNA- and protein analysis as well as single-cell technologies. Focus on ultrasensitive sequencing, especially SiMSen-Seq. Biobank Blood, plasma, urine Key publications Johansson G, Kaltak M, Rîmniceanu C, Singh AK, Lycke J, Malmeström C, Hühn M, Vaarala O, Cardell S, Ståhlberg A. Ultrasensitive DNA immune repertoire sequencing using unique molecular identifiers. Clinical Chemistry, 2020, 20:hvaa159.
Andersson D, Fagman H, Dalin M, Ståhlberg A. Circulating cell-free tumor DNA analysis in pediatric cancers. Molecular Aspects of Medicine, 2020, 72:100819.
Johansson G, Andersson D, Filges S, Li J, Muth A, Godfrey TE, Ståhlberg, A. Considerations and quality controls when analyzing cell-free tumor DNA. Biomolecular Detection and Quantification, 2019, 17:100078.
Ståhlberg A, Krzyzanowski PM, Egyud M, Filges S, Stein L, Godfrey TE. Simple multiplexed PCR-based barcoding of DNA for ultra-sensitive mutation detection by next-generation sequencing (SiMSen-Seq). Nature Protocols, 2017, 12:664-82.
Ståhlberg A, Krzyzanowski PM, Jackson JB, Egyud M, Stein L, Godfrey TE. Simple, multiplexed, PCR-based barcoding of DNA enables sensitive mutation detection in liquid biopsies using sequencing. Nucleic Acids Research, 2016, 44:e105
- University of Patras
- ELBS Team
- Profile Liquid Biopsy Research
-
University of Patras


University of Patras was founded in 1964. It has 3.931 Postgraduate students, 35 Departments, 161 Laboratories and 17 Clinics, The Department of Biology was founded in 1967 and is the first Department of Biology founded in Greece. The Department provides studies on all aspects of Biological science. The research activities of the members of the department cover a wide range of cutting-edge biological technologies. The Department of Biology is housed in a building of 25000 sq.m. and has a significant infrastructure of scientific equipment distributed in educational and research laboratories.
The Laboratory of Biochemistry/Liquid Biopsy situated in the Department of Biology is focused on the Evaluation of signal transduction pathways in Cancer Cells. Enumeration and Characterization of Circulating and Disseminated Tumor Cells (CTCs/DTCs). Investigation of the crosstalk between CTCs and Immune Cells. Development of innovative diagnostic tools for the detection of circulating tumor cells (CTCs) in patients' blood. Assessment of tailor-made substrates for three-dimensional (3D) cultures of tumor cells and CTCs to test drug efficacy (laser-made 3D scaffolds induced on natural biopolymer films). Participation in the development of hyperspectral artificial vision-guided robotic microscope for fixed cells and live cell imaging (CTCs).
-
-
ELBS Team

Galatea Kallergi, Msc, PhD
Assistant Professor of Biochemistry, Division of Genetics, Cell and Developmental Biology
Department of Biology
University of Patras
26504 Patras
Greecegalkallergi@gmail.com
-
-
Profile Liquid Biopsy Research
Cancer types Breast Cancer, Colon Cancer, NSCLC, SCLC etc Clinical application Identification of new biomarkers and therapeutic targets on CTCs, Exosomes etc. Development of new methods for culturing and imaging CTCs for testing drug efficacy. Liquid Biopsy source Blood, plasma, Bone marrow Technologies available for Liquid Biopsy High Resolution Image Analysis systems (ZEISS Axioscope), Transmission Electron Microscope (TEM), Scanning Electron Microscope (SEM), Real Time -PCR etc Biobank Isolated CTCs from patients with solid tumors, ISET Filters with CTCs from NSCLC and Breast Cancer patients. Key publications G. Kallergi, O. Hoffmann, AK. Bittner, L. Papadimitriou, N Zacharopoulou, M. Zervakis, S. Sfakianakis, C. Stournaras, V. Georgoulias, R. Kimmig, S. Kasimir-Bauer. CXCR4 and JUNB double positive disseminated tumor cells are frequently detected in breast cancer patients at primary diagnosis (Under press in Therapeutic Advances in Medical Oncology).
G. Kallergi, V. Tsintari, S Sfakianakis, E.S. Bei, N. Zacharopoulou, C. Stournaras, M. Zervakis and V. Georgoulias The prognostic value of JUNB-positive CTCs in metastatic breast cancer; from bioinformatics to immunophenotype. Breast Cancer Res. 2019 Aug 1;21(1):86..
G. Kallergi, D. Aggouraki, N. Zacharopoulou, C. Stournaras, V. Georgoulias, S.S. Martin. Evaluation of microtentacles on Circulating Tumor Cells (CTCs); Interaction between CTCs and blood cells through cytoskeletal proteins. Breast Cancer Res. 2018 Jul 5;20(1):67.
G, Kallergi, E.K. Vetsika, D. Agouraki, E. Lagoudaki, A. Koutsopoulos, F. Koinis , P. Katsarlinos, M. Trypaki, I. Messaritakis, C. Stournaras, V. Georgoulias, A. Kotsakis. Evaluation of PD-L1/PD-1 on Circulating Tumor Cells (CTCs) in patients with advanced non-small cell lung cancer (NSCLC). Therapeutic Advances in Medical Oncology Journal, 2018 Jan 15;10.
Agelaki, Melina Dragolia, H. Markonanolaki, S. Alkahtani, Ch. Stournaras, V. Georgoulias, G. Kallergi, Phenotypic characterization of circulating tumor cells (CTCs) in triple negative breast cancer patients (TNBC), Oncotarget 2017 Jan 17;8(3):5309-5322.
- University of Trento
- ELBS Team
- Profile Liquid Biopsy Research
-
University of Trento


The University of Trento (UniTN) is a research-oriented medium-sized top-ranked Italian University. It ranked 1st in the 2023/2024 Censis ranking, 1st for scientific output in 2016 and 2022 ANVUR/VQR, and classified 506th among the 1,500 universities listed in QS World University Rankings 2025. The Department of Cellular, Computational and Integrative Biology (DCIBIO, https://www.cibio.unitn.it/ ) at UniTN is a cutting-edge academic department in the biomedicine and biotechnology field, hosting more than 40 national and international PIs. The DCIBIO’s infrastructure provides state-of-the-art core facilities for next-generation sequencing, flow cytometry, advanced imaging, high throughput screening, proteomics, animal modeling, cell technology, and bioinformatics services. In 2023, DCIBIO was recognized as a Department of Excellence by the Italian Ministry of University and Research.
Within DCIBIO, there is a liquid biopsy community that includes multiple PIs running translational projects focusing on solid cancers and neurodegenerative diseases. By integrating molecular and computational biology and through tight collaborations with numerous national and international clinical partners, the research teams combine specific expertise in cell-free DNA (cfDNA) and extracellular vesicles (EVs). Investigations span the study of diseases’ biology, the identification of biomarkers of disease diagnosis, monitoring, prognosis, and treatment response, and technology development.
-
-
ELBS Team

Francesca Demichelis
Professor, Principal Investigator
Laboratory of Computational and Functional Oncology
Department of Cellular, Computational and Integrative Biology
University of TrentoVia Sommarive, 9
38123 Trento – Italy
Manuela Basso
Professor, Principal Investigator
Laboratory of Transcriptional Neurobiology
Department of Cellular, Computational and Integrative Biology
University of TrentoVia Sommarive, 9
38123 Trento – Italy
Vito D’Agostino
Professor, Principal Investigator
Laboratory of Biotechnology and nanomedicine
Department of Cellular, Computational and Integrative Biology
University of TrentoVia Sommarive, 9
38123 Trento – Italy
Caterina Nardella
Dr., Senior Scientist and Programme Manager
Laboratory of Computational and Functional Oncology
Department of Cellular, Computational and Integrative Biology
University of TrentoVia Sommarive, 9
38123 Trento – Italy
Valentina Adami
Dr., Core Facilities Coordinator
Department of Cellular, Computational and Integrative Biology
University of TrentoVia Sommarive, 9
38123 Trento – Italy
Fabio Ledda
Dr., Head, Strategic Research Programmes Division
University of TrentoVia Verdi, 8
38122 Trento – Italy -
-
Profile Liquid Biopsy Research
Cancer types Solid cancers, including prostate, bladder, breast, lung, and melanoma. Other diseases: Amyotrophic lateral sclerosis (ALS). Clinical application Early disease detection, diagnosis, monitoring, and prognosis; treatment response prediction and monitoring; detection of minimal residual disease. Liquid Biopsy Source Plasma, serum, urine, cerebrospinal fluid. Technologies available for liquid biopsy Instruments: NovaSeq6000 and MiSeq (Illumina), ZetaView® NTA (Particle Metrix), NanoSight NS300 (Malvern Panalytical), qNano Gold (Izon Science), BD FACSSymphony A1 (BD Bioscences), ImageStream MarkII (Luminex Corporation), QX200™ Droplet Digital PCR System (Bio-Rad), TapeStation 4150 (Agilent), ONI Nanoimager, Leica TCS SP8 laser scanning confocal system with LIGHTENING module, confocal spinning disc Nikon Eclipse Ti2 + CrestOptics Xlight V2, Nikon microscope for super-resolution SIM combined with confocal laser scanning microscopy. Other: In-house developed targeted assays for ctDNA detection based on mutational profiles and allele-specific genomic signal or methylation. Biobank Ongoing establishment of an EV-specfic biobank for highly annotated biofluids, including plasma and cerebrospinal fluid from patients with solid cancers or neurodegenarative diseases. This biobank will be included in the BBMRI.it biobank network (https://www.bbmri.it/en/), the Italian node of BBMRI-ERIC, the European research infrastructure for biobanking.
Other: Solid tumors and ALS samples - plasma, serum, urine, and cerebrospinal fluid from patients’ cohorts are banked at DCIBIO as part of specific approved study protocols.Key publications Basso M, Gori A, Nardella C, Palviainen M, Holcar M, Sotiropoulos I, Bobis-Wozowicz S, D'Agostino VG, Casarotto E, Ciani Y, Suetsugu S, Gualerzi A, Martin-Jaular L, Boselli D, Kashkanova A, Parisse P, Lippens L, Pagliuca M, Blessing M, Frigerio R, Fourniols T, Meliciano A, Fietta A, Fioretti PV, Soroczyńska K, Picciolini S, Salviano-Silva A, Bergese P, Zocco D, Chiari M, Jenster G, Waldron L, Milosavljevic A, Nolan J, Monopoli MP, Witwer KW, Bussolati B, Di Vizio D, Falcon Perez J, Lenassi M, Cretich M, Demichelis F. International Society for Extracellular Vesicles Workshop. QuantitatEVs: multiscale analyses, from bulk to single extracellular vesicle. J Extracell Biol. 2024 Jan;3(1):e137. doi: 10.1002/jex2.137. Epub 2024 Jan 24. PMID: 38405579.
Franceschini GM, Quaini O, Mizuno K, Orlando F, Ciani Y, Ku SY, Sigouros M, Rothmann E, Alonso A, Benelli M, Nardella C, Auh J, Freeman D, Hanratty B, Adil M, Elemento O, Tagawa ST, Feng FY, Caffo O, Buttigliero C, Basso U, Nelson PS, Corey E, Haffner MC, Attard G, Aparicio A, Demichelis F#, Beltran H#. Noninvasive Detection of Neuroendocrine Prostate Cancer through Targeted Cell-free DNA Methylation. Cancer Discov. 2024 Mar 1;14(3):424-445. doi: 10.1158/2159-8290.CD-23-0754. PMID: 38197680. #Equally contributed.
Mugoni V, Ciani Y, Quaini O, Tomasini S, Notarangelo M, Vannuccini F, Marinelli A, Leonardi E, Pontalti S, Martinelli A, Rossetto D, Pesce I, Mansy SS, Barbareschi M, Ferro A, Caffo O, Attard G, Di Vizio D, D'Agostino VG#, Nardella C#, Demichelis F#. Integrating extracellular vesicle and circulating cell-free DNA analysis using a single plasma aliquot improves the detection of HER2 positivity in breast cancer patients. J Extracell Biol. 2023 Sep;2(9):e108. doi: 10.1002/jex2.108. Epub 2023 Sep 25. PMID: 38046436. #Equally contributed.
Orlando F, Romanel A, Trujillo B, Sigouros M, Wetterskog D, Quaini O, Leone G, Xiang JZ, Wingate A, Tagawa S, Jayaram A, Linch M; PEACE Consortium; Jamal-Hanjani M, Swanton C, Rubin MA, Wyatt AW, Beltran H, Attard G, Demichelis F. Allele-informed copy number evaluation of plasma DNA samples from metastatic prostate cancer patients: the PCF_SELECT consortium assayNAR Cancer. 2022 May 27;4(2):zcac016. doi: 10.1093/narcan/zcac016. eCollection 2022 Jun. PMID: 35664542.
Pasetto L, Callegaro S, Corbelli A, Fiordaliso F, Ferrara D, Brunelli L, Sestito G, Pastorelli R, Bianchi E, Cretich M, Chiari M, Potrich C, Moglia C, Corbo M, Sorarù G, Lunetta C, Calvo A, Chiò A, Mora G, Pennuto M, Quattrone A, Rinaldi F, D'Agostino VG, Basso M#, Bonetto V#. Decoding distinctive features of plasma extracellular vesicles in amyotrophic lateral sclerosis. Mol Neurodegener. 2021 Aug 10;16(1):52. doi: 10.1186/s13024-021-00470-3. PMID: 34376243. #Equally contributed.
- Veneto Institute of Oncology IOV – IRCCS
- ELBS Team
- Profile Liquid Biopsy Research
-
Veneto Institute of Oncology IOV – IRCCS


The Veneto Institute of Oncology IOV-IRCCS, is a public comprehensive cancer center offering a wide array of specialized services. Our mission encompasses research, prevention, diagnosis, and cutting-edge treatments, spanning from standard to experimental modalities. With a dedicated focus on all types of oncologic diseases, including rare conditions, we provide holistic care to patients at every stage of their journey. Our multidisciplinary approach ensures that patients with primary, recurrent, or metastatic conditions receive personalized care aligned with national and international guidelines. Through regular multidisciplinary meetings, our expert teams collaborate to tailor treatment plans to individual needs.
Outpatient services reach nearly 130.000 patients per year, both from Veneto Region and from the rest of Italy. Every year more than 6.700 surgical operations are performed and more than 10.000 patients are treated with radiotherapy or systemic therapy.
Beyond clinical excellence, we are committed to advancing the field through rigorous research. Our teams actively engage in basic and translational research, exploring biology, predictive biomarkers, and drug development. The Institute coordinates the preparation of treatment pathways at the regional level. Moreover, we play a pivotal role in shaping national clinical guidelines through our partnership with the Italian Association of Medical Oncology (AIOM). Collaboration lies at the heart of our efforts. IOV is also part of Italian Lung Screening Network, the ongoing programme of the Ministry of Health for the early diagnosis of lung cancer with low-dose computed chest tomography. We work closely with esteemed European and international cancer organizations, as well as pharmaceutical companies, to ensure patients have access to the most innovative therapies and resources. Recognition from prestigious bodies, such as the Organization of European Cancer Institutes (OECI) and the European Society of Medical Oncology (ESMO), underscores our commitment to excellence.
-
-
ELBS Team
Dr. Maria Chiara Scaini, PhD
Researcher
Immunology and molecular oncology unit – IOVCorso Stati Uniti, 4
35127 Padova
ITALYProf. Stefano Indraccolo, M.D.
Associate professor – University of Padova
Director – Basic and translational oncology unit – IOVVia Gattamelata, 64
35128 Padova
ITALY
-
Profile Liquid Biopsy Research
Cancer types Solid tumors (lung, breast, melanoma, bladder, gastroesophageal cancer) Clinical application Identification of markers of response to immunotherapy and target therapy. Liquid Biopsy Source blood, plasma, urine Technologies available for liquid biopsy NGS (Illumina and ThermoFisher, ddPCR (Biorad), CellSearch Biobank Solid tumors (plasma, serum) Key publications A multiparameter liquid biopsy approach allows to track melanoma dynamics and identify early treatment resistance. Scaini MC, Catoni C, Poggiana C, Pigozzo J, Piccin L, Leone K, Scarabello I, Facchinetti A, Menin C, Elefanti L, Pellegrini S, Aleotti V, Vidotto R, Schiavi F, Fabozzi A, Chiarion-Sileni V, Rosato A.
NPJ Precis Oncol 2024 Mar 28;8(1):78. doi: 10.1038/s41698-024-00567-0.
Putative Clinical Potential of ERBB2 Amplification Assessment by ddPCR in FFPE-DNA and cfDNA of Gastroesophageal Adenocarcinoma Patients. Boldrin E, Mazza M, Piano MA, Alfieri R, Montagner IM, Magni G, Scaini MC, Vassallo L, Rosato A, Pilati P, Scapinello A, Curtarello M.
Cancers (Basel) 2022 Apr 27;14(9):2180. doi: 10.3390/cancers14092180.
Longitudinal liquid biopsy anticipates hyperprogression and early death in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors.
Zulato E, Del Bianco P, Nardo G, Attili I, Pavan A, Boscolo Bragadin A, Marra L, Pasello G, Fassan M, Calabrese F, Guarneri V, Conte PF, Indraccolo S, Bonanno L.
Br J Cancer. 2022 Nov;127(11):2034-2042. doi: 10.1038/s41416-022-01978-1.
Early assessment of KRAS mutation in cfDNA correlates with risk of progression and death in advanced non-small-cell lung cancer. Zulato E, Attili I, Pavan A, Nardo G, Del Bianco P, Boscolo Bragadin A, Verza M, Pasqualini L, Pasello G, Fassan M, Calabrese F, Guarneri V, Amadori A, Conte P, Indraccolo S, Bonanno L.
Br J Cancer. 2020 Jul;123(1):81-91. doi: 10.1038/s41416-020-0833-7.
Prognostic Stratification of Bladder Cancer Patients with a MicroRNA-based Approach. Cavallari I, Grassi A, Del Bianco P, Aceti A, Zaborra C, Sharova E, Bertazzolo I, D'Agostino DM, Iafrate M, Ciminale V.
Cancers (Basel). 2020 Oct 26;12(11):3133. doi: 10.3390/cancers12113133.


