Statistical methods for the analysis of multiple time to event endpoints
In medical research, clinical trials are usually used to demonstrate the efficacy or effectiveness of a drug, agent, or treatment. This evidence should be based on a so-called "hard" endpoint. These include patient-relevant endpoints such as death, in cardiology studies, for example, also ischemic stroke, specific types of death such as cardiovascular death, or hospitalization for reasons such as the occurrence of acute coronary syndrome. The time to occurrence of any of these events is often of primary interest, i.e., time to event analysis.
However, it can take a long time until one of these endpoints is observed due to the well effective standard care. This considerably prolongs the duration of a study, which is thus often not feasible for cost reasons. A large number of patients is usually required to demonstrate a relevant effect of the new treatment for sufficient power. Depending on the clinical situation under consideration, this number of patients can hardly be realized in a clinical study within a reasonable time.
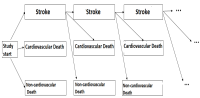
In order to overcome this problem and still be able to demonstrate efficacy in a study with a clinically relevant endpoint, so-called composite endpoints can be used or/and all events per patient:in can be considered. Methods for planning and analyzing studies with such multiple time to event endpoints need to be evaluated in more detail. This is done within a DFG-funded project.