AG Mohme ("CNS Tumor Immunology")
Tumors of the central nervous system pose a particular challenge to interdisciplinary oncological research and therapy. First and foremost is glioblastoma, which is the most aggressive primary tumor of the central nervous system. The infiltrative growth of glioblastoma cells into the surrounding tissue generally makes complete surgical resection impossible and the risk of consecutive recurrence is high. New treatment approaches that are able to seek out, identify and specifically destroy tumor cells are urgently needed. Immunotherapies offer these possibilities.
The interaction between tumor cells and the immune system is already of great relevance in tumor development. Early studies have shown that a defective immune system is no longer able to eliminate mutated or aberrant cells in early tumor development. It is therefore assumed that an intact immune system contributes significantly to the continuous destruction of developing tumor cells even before clonal expansion. This means that tumor development is only possible if a tumor manages to escape the immune system through specific mutations or by acquiring immunosuppressive mechanisms. The aim of tumor immunotherapies is to reactivate the patient's own adaptive immune system or to switch off the tumor invasion mechanisms in order to enable specific tumor elimination. Numerous studies have already shown initial success with tumor immunological therapy approaches. Above all, studies in the field of prostate carcinoma and malignant melanoma. Both vaccination with tumor antigens in the form of peptides and the transfer of tumor-specific T cells or tumor antigen-presenting dendritic cells were able to induce a specific reaction of the immune system against these tumors. This means that the patient's own adaptive immune system can be specifically activated against the tumor cells and thus the intrinsic cytotoxic mechanisms of the immune cells are used to eliminate tumor cells. Similar observations have been made in many other tumor entities, including multiple myeloma, renal cell carcinoma, breast carcinoma, colon carcinoma, lung carcinoma, etc. Initial promising results have also been obtained in the field of malignant brain tumors. The number of tumor-infiltrating immune cells and the strength of the immune response to so-called tumor antigens correlate with the overall survival of patients. "Tumor vaccination studies" in phase I/II have shown good tolerability and the elicitation of a tumor-specific immune response. In mouse models, it was even possible to completely cure solid tumors with vaccinations and induce persistent tumor immunity. Although effective and potentially curative immunotherapy is possible, previous studies have shown that malignant gliomas evade the immune system through two basic mechanisms. First, by manipulation of the local tumor environment, including the evolution of so-called immune escape mechanisms, and second, by their heterogeneity, which makes it difficult for the immune system to recognize the tumor cells. Summarizing literature can be found under the following references.
Research Focus
- Immune phenotyping of tumor-specific T cell responses (Flow cytometry)
- Clonal repertoire dynamics in malignant brain tumors (TCR seq)
- Epigenetic modulation of T cells in gliomas (EPIC, ATACseq, CHIPseq)
- Impact of viral antigens on tumor-specific T cell activation
Funding
Group Members
- Dr. rer. nat. Krystian Fita (PostDoc)
- Dr. med. univ. Alice Senta Ryba (Clinician Scientist)
- Alessandra Rünger (Clinician Scientist)
- Rebecca Kringel (Clinician Scientist)
- Merle Reetz (Doctoral Student)
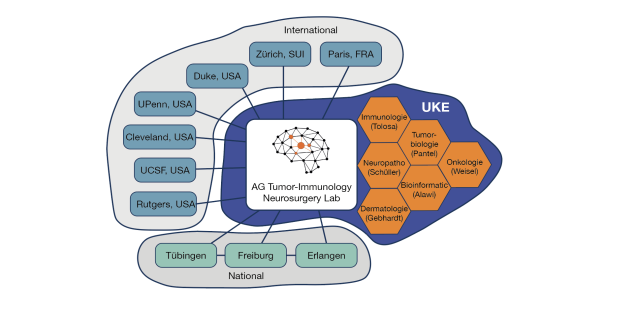
Collaborations
Selected Publications
Mohme M, Schultheiß C, Piffko A, Fitzek A, Paschold L, Thiele B, Püschel K, Glatzel M, Westphal M, Lamszus K, Matschke J, Binder M. SARS-CoV-2-associated T-cell infiltration in the central nervous system. Clin Transl Immunology. 2024 Jan 31;13(2):e1487. doi: 10.1002/cti2.1487. eCollection 2024. PMID: 38304555
Drexler R, Brembach F, Sauvigny J, Ricklefs FL, Eckhardt A, Bode H, Gempt J, Lamszus K, Westphal M, Schüller U, Mohme M. Unclassifiable CNS tumors in DNA methylation-based classification: clinical challenges and prognostic impact. Acta Neuropathol Commun. 2024 Jan 16;12(1):9. doi: 10.1186/s40478-024-01728-9. PMID: 3822915
Kringel R, Lamszus K, Mohme M. Chimeric Antigen Receptor T Cells in Glioblastoma-Current Concepts and Promising Future. Cells. 2023 Jul 3;12(13):1770. doi: 10.3390/cells12131770. PMID: 37443804
Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma. Naghavian R, Faigle W, Oldrati P, Wang J, Toussaint NC, Qiu Y, Medici G, Wacker M, Freudenmann LK, Bonté PE, Weller M, Regli L, Amigorena S, Rammensee HG, Walz JS, Brugger SD, Mohme M, Zhao Y, Sospedra M, Neidert MC, Martin R. Nature. 2023 May 17. PMID: 37198490
Yang Y, Brown MC, Zhang G, Stevenson K, Mohme M, Kornahrens R, Bigner DD, Ashley DM, López GY, Gromeier M. Polio virotherapy targets the malignant glioma myeloid infiltrate with diffuse microglia activation engulfing the CNS. Neuro Oncol. 2023 Sep 5;25(9):1631-1643. doi: 10.1093/neuonc/noad052. PMID: 36864784
Piffko A, Asey B, Dührsen L, Ristow I, Salamon J, Wikman H, Maire CL, Lamszus K, Westphal M, Sauvigny T, Mohme M.Clinical determinants impacting overall survival of patients with operable brain metastases from non-small cell lung cancer. Front Oncol. 2022 Oct 20. PMID: 36338751
Monteiro C, Miarka L, Perea-García M, Priego N, García-Gómez P, Álvaro-Espinosa L, de Pablos-Aragoneses A, Yebra N, Retana D, Baena P, Fustero-Torre C, Graña-Castro O, Troulé K, Caleiras E, Tezanos P, Muela P, Cintado E, Trejo JL, Sepúlveda JM, González-León P, Jiménez-Roldán L, Moreno LM, Esteban O, Pérez-Núñez Á, Hernández-Lain A, Mazarico Gallego J, Ferrer I, Suárez R, Garrido-Martín EM, Paz-Ares L, Dalmasso C, Cohen-Jonathan Moyal E, Siegfried A, Hegarty A, Keelan S, Varešlija D, Young LS, Mohme M, Goy Y, Wikman H, Fernández-Alén J, Blasco G, Alcázar L, Cabañuz C, Grivennikov SI, Ianus A, Shemesh N, Faria CC, Lee R, Lorigan P, Le Rhun E, Weller M, Soffietti R, Bertero L, Ricardi U, Bosch-Barrera J, Sais E, Teixidor E, Hernández-Martínez A, Calvo A, Aristu J, Martin SM, Gonzalez A, Adler O, Erez N; RENACER; Valiente M. Stratification of radiosensitive brain metastases based on an actionable S100A9/RAGE resistance mechanism. Nature Medicine. 2022, PMID: 35411077
Simnica D*, Schultheiß C*, Mohme M*, Paschold L, Willscher E, Fitzek A, Püschel K, Matschke J, Ciesek S, Sedding DG, Zhao Y, Gagliani N, Maringer Y, Walz JS, Heide J, Schulze-Zur-Wiesch J, Binder M. Landscape of T-cell repertoires with public COVID-19-associated T-cell receptors in pre-pandemic risk cohorts. Clin Transl Immunology. 2021 Aug 28 PMID: 34484739
Safaei S, Mohme M, Niesen J, Schüller U*, Bockmayr M*, DIMEimmune: Robust estimation of infiltrating lymphocytes in CNS tumors from DNA methylation profiles, Oncoimmunol, Jun 2021, PMID: 34235002
Brown M, Mosaheb M, Mohme M, McKay Z, Holl E, Kastan J, Yang Y, Beasley G, Hwang E, Ashley D, Bigner D, Nair S, Gromeier M, Viral infection of cells within the tumor microenvironment mediates antitumor immunotherapy via selective TBK1-IRF3 signaling, Nat Comm, Mar 2021, PMID: 33767151
Maire CL*, Mohme M*, Bockmayr M, Fita KD, Riecken K, Börnigen D, Alawi M, Failla A, Kolbe K, Zapf S, Holz M, Neumann K, Dührsen L, Lange T, Fehse B, Westphal M, Lamszus K, Glioma escape signature and clonal development under immune pressure, J Clin Invest, Oct 2020, PMID: 32603315
Mohme M, Maire CL, Schliffke S, Joosse SA, Alawi M, Matschke J, Schüller U, Dierlamm J, Martens T, Pantel K, Riethdorf S, Lamszus K, Westphal M, Molecular profiling of an osseous metastasis in glioblastoma during checkpoint inhibition: Potential mechanisms of immune escape, Acta Neuropath Comm, Mar 2020, PMID: 32151286
Mohme M, Neidert MC, Tumor-specific T cell activation in malignant brain tumors, Front Immunol, Feb 2020, PMID: 32117316
Mohme M*, Maire CL*, Geumann U, Schliffke S, Dührsen L, Fita K, Akyüz N, Binder M, Westphal M, Guenther C, Lamszus K, Hermann FG, Schmidt NO, Local intracerebral immunomodulation using interleukin-expressing mesenchymal stem cells in glioblastoma: preclinical studies, Clin Canc Res, Jun 2020, PMID: 31988196
Mohme M*, Sauvigny T*, Mader M, Schweingruber N, Maire CL, Rünger A, Ricklefs R, Regelsberger J, Schmidt NO, Westphal M, Lamszus K, Tolosa E*, Czorlich P*, Immune characterization in aneurysmal subarachnoid hemorrhage reveals distinct monocytic activation and chemokine patterns, Trans Stroke Res, Dec 2020, PMID: 31858408
Bockmayr M, Klauschen F, Maire CL, Rutkowski S, Westphal M, Lamszus K, Schüller U*, Mohme M*, Immunologic Profiling of Mutational and Transcriptional Subgroups in Pediatric and Adult High-Grade Gliomas, Canc Immunol Res, Sep 2019, PMID: 31266783, do: 10.1158/2326-6066.CIR-18-0939
Mohme M, Maire CL, Riecken K, Zapf S, Aranyossy T, Westphal M, Lamszus K, Fehse B., Optical Barcoding for Single-Clone Tracking to Study Tumor Heterogeneity. Mol Ther. 2017 Jan 18. pii: S1525-0016(16)45496-1. doi: 10.1016/j.ymthe.2016.12.014.] PMID: 28109958
Mohme M, Riethdorf S, Pantel K., Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2016 Sep 20. doi: 10.1038/nrclinonc.2016.144. Review. PMID: 27644321
Mohme, M., Neidert, M. C., Regli, L., Weller, M. & Martin, R. Immunological challenges for peptide-based immunotherapy in glioblastoma. Cancer Treatment Reviews 40, 248–258 (2014).
Westphal, M. & Lamszus, K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat. Rev. Neurosci. 12, 495–508 (2011). Muller, C. et al. Hematogenous dissemination of glioblastoma multiforme. Sci. Transl. Med. 6, 247ra101–247ra101 (2014).
Pantel K, Alix-Panabieres C. The clinical significance of circulating tumor cells. Nat Clin Pract Oncol. 2007;4:62-3.